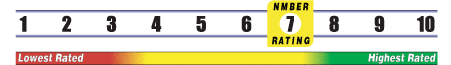
| Ingredients | Amount Per Serving |
|---|---|
|
(Isoflavones)
|
150 mg |
|
(seed)
(Lignans)
|
120 mg |
|
(husk)
(Ellagic Acid)
|
100 mg |
Flax seed powder PlantPart: seed, Vegetable Hypromellose, Silica, Maltodextrin
Below is general information about the effectiveness of the known ingredients contained in the product Phytoestrogen Essential Complex. Some ingredients may not be listed. This information does NOT represent a recommendation for or a test of this specific product as a whole.
INSUFFICIENT RELIABLE EVIDENCE to RATE
INSUFFICIENT RELIABLE EVIDENCE to RATE
INSUFFICIENT RELIABLE EVIDENCE to RATE
Below is general information about the safety of the known ingredients contained in the product Phytoestrogen Essential Complex. Some ingredients may not be listed. This information does NOT represent a recommendation for or a test of this specific product as a whole.
LIKELY SAFE ...when ground flaxseed is used orally and appropriately. Ground flaxseed has been safely used in numerous clinical trials in doses up to 30-60 grams daily for up to 1 year (6803,6808,8020,10952,10978,12908,12910) (16760,16761,16762,16765,16766,18224,21191,21194,21196,21198) (21199,21200,22176,22179,22180,22181,65866,66065) (101943,101949,101950).
POSSIBLY SAFE ...when flaxseed lignan extract or mucilage is used orally and appropriately. Some clinical research shows that a specific flaxseed lignan extract (Flax Essence, Jarrow Formulas) 600 mg daily can be used with apparent safety for up to 12 weeks (16768). Additional clinical research shows that other flaxseed lignin extracts can be used with apparent safety for up to 6 months (21193,21197,21200). In one clinical trial, flaxseed mucilage was used with apparent safety at a dose of up to 5120 mg daily for up to 12 weeks (108047)....when flaxseed is used topically in a warm poultice (101946).
POSSIBLY UNSAFE ...when raw or unripe flaxseed is used orally. Raw flaxseed contains potentially toxic cyanogenic glycosides (linustatin, neolinustatin, and linamarin); however, these glycosides have not been detected after flaxseed is baked (5899). Unripe flaxseeds are also thought to be poisonous when consumed due to cyanide content.
PREGNANCY: POSSIBLY UNSAFE
when used orally.
Flaxseed can have mild estrogenic effects. Theoretically, this might adversely affect pregnancy (9592,12907); however, there is no reliable clinical evidence about the effects of flaxseed on pregnancy outcomes.
LACTATION:
Insufficient reliable information available; avoid using.
LIKELY SAFE ...when pomegranate fruit or fruit juice is used orally and appropriately. Pomegranate juice has been safely used in studies lasting up to 3 years (4912,8310,13022,13023,13690,14137,14388,17329,91693).
POSSIBLY SAFE ...when pomegranate extract is taken orally and appropriately. A specific pomegranate ellagitannin-enriched polyphenol extract (POMx, POM Wonderful) 1-3 grams daily has been safely used for up to 18 months (17729,69261,91686,91695,91697,99100,105269). ...when pomegranate seed oil is used orally and appropriately. Pomegranate seed oil 60 mg daily has been used with apparent safety for up to 12 weeks (91685). ...when a hot water extract of pomegranate seed powder is used orally and appropriately. Pomegranate seed powder 5 grams daily has been used with apparent safety for up to 8 weeks (105270). ...when pomegranate extract is used topically on oral mucosa (13689).
POSSIBLY UNSAFE ...when the pomegranate root, stem, and peel are used orally in large amounts. Bark of the pomegranate root and stem contains the piperidine alkaloids pelletierine, pseudopelletierine, isopelletierine, and methyl isopelletierine. These alkaloids have muscle relaxant properties that have been associated with paralysis and death in animals (13687,13694,13695). Dried pomegranate peel may contain aflatoxin, which is a potent hepatocarcinogen and toxin (92018).
PREGNANCY AND LACTATION: POSSIBLY SAFE
when the fruit or fruit juice is consumed orally and appropriately (13686,105267).
There is insufficient reliable information available regarding the safety of using other forms of pomegranate or other parts of the plant during pregnancy or lactation; avoid using.
LIKELY SAFE ...when soy protein is used orally and appropriately. Soy protein products in doses up to 60 grams, providing up to 185 mg isoflavones, daily have been safely used in studies lasting up to 16 weeks (842,2293,2294,2296,3025,3402,3977,4755,6412,8530)(10372,11805).
POSSIBLY SAFE ...when soy extracts are used orally and appropriately, short-term. Soy extracts containing concentrated isoflavones in doses of 35-120 mg daily have been used with apparent safety for up to 6 months (4751,6455,7802,12040,12048,13209,95994,95999).
CHILDREN: LIKELY SAFE
when consumed in amounts commonly found in foods or as a component of infant formula (3400,4912,7331).
Soy milk that's not designed for infants should not be used as a substitute for infant formula. Regular soy milk can lead to nutrient deficiencies (12045). Most evidence shows that exposure to soy formula or other soy products in infancy does not cause early onset of puberty or health or reproductive problems later in life (7331,11080,108245). However, some small cohort studies have suggested that higher soy intake during childhood may be associated with an increased risk of precocious puberty (108240) and may be weakly correlated with the development of breasts in children less than 2 years of age (75520). This is in contrast to an observational study in Chinese children ages 7-9 years which suggests that higher soy intake is associated with delayed puberty (108252). One small cohort study has also found that use of soy infant formula may be associated with an increased risk of endometriosis in adulthood, although endometriosis was also correlated with prematurity, which may have confounded the findings (101803).
CHILDREN: POSSIBLY UNSAFE
when used orally as an alternative to cow's milk in children with severe milk allergy (75359).
Although soy protein-based infant formulas are often promoted for children with milk allergy, children with a severe allergy to cow's milk are also frequently sensitive to soy protein (9883). There is insufficient reliable information available about the safety of soy products when used in amounts higher than typical food quantities for children.
PREGNANCY: LIKELY SAFE
when used orally in amounts commonly found in foods (4912).
PREGNANCY: POSSIBLY UNSAFE
when used orally in medicinal amounts.
Soy contains mildly estrogenic constituents (3373,3988,3989,3990,3994,6029,75303). Theoretically, therapeutic use of soy might adversely affect fetal development; avoid using.
LACTATION: LIKELY SAFE
when used orally in amounts commonly found in foods (4912).
A single 20-gram dose of roasted soybeans, containing 37 mg isoflavones, produces four to six times less isoflavones in breast milk than provided in a soy-based infant formula (2290). There is insufficient reliable information available about the safety of long-term use of therapeutic amounts of soy during lactation.
Below is general information about the interactions of the known ingredients contained in the product Phytoestrogen Essential Complex. Some ingredients may not be listed. This information does NOT represent a recommendation for or a test of this specific product as a whole.
Theoretically, antibiotics might interfere with the metabolism of flaxseed constituents, which could potentially alter the effects of flaxseed.
Some potential benefits of flaxseed are thought to be due to its lignan content. Secoisolariciresinol diglucoside (SDG), a major lignan precursor, is found in high concentrations in flaxseed. SDG is converted by bacteria in the colon to the lignans enterolactone and enterodiol (5897,8022,8023,9592). Antibiotics alter the flora of the colon, which could theoretically alter the metabolism of flaxseed.
|
Theoretically, using flaxseed in combination with anticoagulant or antiplatelet drugs might have additive effects and increase the risk of bleeding.
|
Theoretically, flaxseed might have additive effects when used with antidiabetes drugs and increase the risk for hypoglycemia.
|
Theoretically, flaxseed might have additive effects when used with antihypertensive drugs and increase the risk of hypotension.
|
Theoretically, taking flaxseed might decrease the effects of estrogens.
Flaxseed contains lignans with mild estrogenic and possible antiestrogenic effects. The lignans seem to compete with circulating endogenous estrogen and might reduce estrogen binding to estrogen receptors, resulting in an anti-estrogen effect (8868,9593). It is unclear if this effect transfers to exogenously administered estrogens.
|
Theoretically, taking pomegranate with ACEIs might increase the risk of adverse effects.
Pomegranate juice is thought to have ACE inhibitor-like effects (8310).
|
Theoretically, taking pomegranate with antihypertensive drugs might increase the risk of hypotension.
|
Theoretically, taking pomegranate with carbamazepine might increase the risk of adverse effects, although research suggests this interaction is unlikely to be clinically significant.
Animal research shows that pomegranate juice may inhibit cytochrome P450 3A4 (CYP3A4) metabolism of carbamazepine and increase levels of carbamazepine by 1.5 times without prolonging the elimination half-life. This suggests that pomegranate juice inhibits intestinal CYP3A4, but might not inhibit hepatic CYP3A4 (13188). However, some human research suggests that pomegranate does not significantly inhibit CYP3A4 drug metabolism in humans (16711,16712,17326).
|
Theoretically, pomegranate might increase levels of drugs metabolized by CYP2C9.
|
Theoretically, pomegranate might increase levels of drugs metabolized by CYP2D6.
In vitro, pomegranate juice inhibits CYP2D6 (13703). However, the clinical significance of this potential interaction in humans is not known.
|
Theoretically, pomegranate might increase levels of drugs metabolized by CYP3A4, but most research suggests this interaction is unlikely to be clinically significant.
Pomegranate contains several polyphenols that have individually been shown to inhibit CYP3A4. However, there is contradictory evidence about the effect of whole pomegranate juice on CYP3A4 activity. In vitro, pomegranate juice significantly inhibits the CYP3A4 enzyme, with comparable inhibition to grapefruit juice (13188,16711,17326). In an animal model, pomegranate juice inhibits CYP3A4 metabolism of carbamazepine and increases levels of carbamazepine by 1.5 times (13188); however, in human volunteers, drinking a single glass of pomegranate juice 240 mL or taking 200 mL daily for 2 weeks does not significantly affect levels of the CYP3A4 substrate midazolam after oral or intravenous administration (16711,17730). Another study in healthy volunteers shows that consuming pomegranate juice 300 mL three times daily for three days also does not significantly affect levels of simvastatin, a CYP3A4 substrate (16712,91696) This suggests that pomegranate is unlikely to significantly affect levels of CYP3A4 substrates in humans (17326).
|
Theoretically, taking pomegranate with rosuvastatin might increase the risk of adverse effects.
In one case, a patient taking rosuvastatin 5 mg every other day in combination with ezetimibe 10 mg daily developed rhabdomyolysis after drinking pomegranate juice 200 mL twice weekly for 3 weeks. This patient had a history of elevated creatine kinase levels while not receiving any statin treatment. This suggests a possible underlying myopathy and predisposition to rhabdomyolysis (14465).
|
Theoretically, pomegranate might increase levels of tolbutamide, although research suggests this interaction is unlikely to be clinically significant.
Animal research shows that pomegranate juice inhibits the cytochrome P450 2C9 (CYP2C9) metabolism of tolbutamide. Pomegranate juice increased tolbutamide levels by 1.2 times without prolonging the elimination half-life. This suggests that pomegranate juice inhibits intestinal CYP2C9, but might not inhibit hepatic CYP2C9 (17327). Despite this evidence, clinical research shows that neither pomegranate juice nor pomegranate extract have a significant effect on CYP2C9 activity in humans (91694). This interaction does not appear to be clinically significant in humans.
|
Theoretically, pomegranate might increase warfarin levels and increase the risk of bleeding. Also, discontinuing regular consumption of pomegranate juice might decrease warfarin levels.
In one case report, a patient had a stable, therapeutic bleeding time, as measured by international normalized ratio (INR), while taking warfarin in combination with pomegranate juice 2-3 times per week. The patient became subtherapeutic within about 10 days after discontinuing pomegranate juice, which required a warfarin dose increase (17328). In another case report, a patient with a stable INR for over one year presented with an INR of 14. The patient noted no changes to medications or diet but did report consuming around 3 liters of pomegranate juice over the previous week. The patient's INR stabilized upon moderation of pomegranate juice consumption (24273). The mechanism of this potential interaction is unclear.
|
Theoretically, antibiotics may decrease the activity of soy isoflavones.
Intestinal bacteria are responsible in part for converting soy isoflavones into their active forms. Antibiotics may decrease the amount of intestinal bacteria and decrease its ability to convert isoflavones (7657).
|
Soy can lower blood glucose and have additive effects with antidiabetes drugs.
Clinical research shows that whole soy diets and soy-based meals reduce fasting glucose levels in diabetic and non-diabetic individuals (75268,75296,75378,75493,96001). Also, individuals following a soy-based meal replacement plan seem to require lower doses of sulfonylureas and metformin to manage blood glucose levels when compared with individuals following a diet plan recommended by the American Diabetes Association (75268).
|
Theoretically soy protein may have additive effects with antihypertensive drugs and increase the risk of hypotension.
|
Theoretically, soy might reduce the clearance of caffeine.
Soy contains genistein. Taking genistein 1 gram daily for 14 days seems to inhibit caffeine clearance and metabolism in healthy females (23582). This effect has been attributed to inhibition of the cytochrome P450 1A2 (CYP1A2) enzyme, which is involved in caffeine metabolism. It is unclear if this effect occurs with the lower amounts of genistein found in soy.
|
Soy might modestly induce CYP2C9 enzymes. However, this effect does not seem to be clinically significant.
In vitro research suggests that an unhydrolyzed soy extract might induce CYP2C9. However, the significance of this interaction is likely minimal. In healthy females taking a specific extract of soy (Genistein Soy Complex, Source Naturals), blood levels of losartan, a CYP2C9 substrate, were not significantly affected (16825).
|
Theoretically, soy might have additive effects when used with diuretic drugs.
Animal research suggests that genistein, a soy isoflavone, increases diuresis within 6 hours of subcutaneous administration in rats. The effects seem to be similar to those of furosemide (75604). This effect has not been reported in humans.
|
Theoretically, soy might competitively inhibit the effects of estrogen replacement therapy.
Soy contains phytoestrogens and has been shown to have estrogenic activity in some patients (3860). Although this has not been demonstrated in humans, theoretically, concomitant use of soy with estrogen replacement therapy might reduce the effects of the estrogen replacement therapy.
|
Soy products might reduce the absorption of levothyroxine in some patients.
Preliminary clinical research and a case report suggest that soy-based formulas inhibit the absorption of levothyroxine in infants with congenital hypothyroidism (20636,20637,75548,90959). A levothyroxine dosage increase may be needed for infants with congenital hypothyroidism while using soy-based formulas, and the dose may need to be reduced when soy-based formulas are no longer administered. However, in postmenopausal adults, clinical research shows that taking a single dose of soy extract containing isoflavones 60 mg along with levothyroxine does not affect the oral bioavailability of levothyroxine (95996).
|
Taking soy products containing high amounts of tyramine along with MAOIs can increase the risk of hypertensive crisis.
Fermented soy products such as tofu and soy sauce contain tyramine, a naturally occurring chemical that affects blood pressure regulation. The metabolism of tyramine is decreased by MAOIs. Consuming more than 6 mg of tyramine while taking an MAOI can increase the risk of hypertensive crisis (15649). The amount of tyramine in fermented soy products is usually less than 0.6 mg per serving; however, there can be significant variation depending on the specific product used, storage conditions, and length of storage. Storing one brand of tofu for a week can increase tyramine content from 0.23 mg to 4.8 mg per serving (15649,15701,15702). Advise patients taking MAOIs to avoid fermented soy products that contain high amounts of tyramine.
|
Theoretically, combining soy isoflavones with transdermal progesterone may worsen bone density.
Clinical research suggests that significant bone loss may occur in females with osteoporosis who receive a combination of transdermal progesterone with soy milk containing isoflavones when compared with placebo, soy milk alone, or progesterone alone (69859).
|
Theoretically, estrogenic soy isoflavones might alter the effects of tamoxifen.
Laboratory research suggests that genistein and daidzen, isoflavones from soy, can antagonize the antitumor effects of tamoxifen under some circumstances (7072,14362,8966); however, soy isoflavones might have different effects when used at different doses. A relatively low in vitro concentration of soy isoflavones such as 1 microM/L seems to interfere with tamoxifen, whereas high in vitro concentrations such as those >10 microM/L might actually enhance tamoxifen effects. People on a high-soy diet have soy isoflavones levels ranging from 0.1-6 microM/L. Until more is known, advise patients taking tamoxifen to avoid therapeutic use of soy products.
|
Theoretically, soy might interfere with the effects of warfarin.
Soy milk has been reported to decrease the international normalized ratio (INR) in a patient taking warfarin. The mechanism of this interaction is not known (9672). However, animal and in vitro research suggests that soy may also inhibit platelet aggregation (3992). Dosing adjustments for warfarin may be necessary.
|
Below is general information about the adverse effects of the known ingredients contained in the product Phytoestrogen Essential Complex. Some ingredients may not be listed. This information does NOT represent a recommendation for or a test of this specific product as a whole.
General
...Orally, flaxseed is usually well-tolerated.
Most Common Adverse Effects:
Orally: Bloating, diarrhea, gastrointestinal complaints.
Serious Adverse Effects (Rare):
Orally: Severe allergic reactions such as and anaphylaxis.
Gastrointestinal
...Integrating flaxseed in the diet can cause digestive symptoms similar to other sources of dietary fiber including bloating, fullness, flatulence, abdominal pain, diarrhea, constipation, dyspepsia, and nausea (12910,16761,16765,21198,21200,22176,22179,65866,101943).
Higher doses are likely to cause more gastrointestinal side effects. Flaxseed can significantly increase the number of bowel movements and the risk for diarrhea (6803,8021,16765). Doses greater than 45 grams per day may not be tolerated for this reason (6802). Metallic aftertaste and bowel habit deterioration have also been reported in a clinical trial (21198).
There is some concern that taking large amounts of flaxseed could result in bowel obstruction due to the bulk forming laxative effects of flaxseed. Bowel obstruction occurred in one patient in a clinical trial (65866). However, this is not likely to occur if flaxseed is consumed with an adequate amount of fluids.
Immunologic ...Occasionally, allergic and anaphylactic reactions have been reported after ingestion of flaxseed (16761). Handling and processing flaxseed products might increase the risk of developing a positive antigen test to flaxseed and hypersensitivity (6809,12911,26471,26482).
Oncologic ...Flaxseed contains alpha-linolenic acid (ALA). High dietary intake of ALA has been associated with increased risk for prostate cancer (1337,2558,7823,7147,12978). However, ALA from plant sources, such as flaxseed, does not seem to increase this risk (12909).
Other ...Orally, partially defatted flaxseed, which is flaxseed with less alpha-linolenic acid, might increase triglyceride levels (6808). Raw or unripe flaxseed contains potentially toxic cyanogenic glycosides (linustatin, neolinustatin, and linamarin). These chemicals can increase blood levels and urinary excretion of thiocyanate in humans. However, these glycosides have not been detected after flaxseed is baked (5899).
General
...Orally, pomegranate fruit juice is generally well tolerated.
Pomegranate fruit extract and seed oil seem to be well tolerated. Pomegranate root, stem, and peel should not be used orally in large amounts. Topically, pomegranate fruit extract seems to be well tolerated.
Most Common Adverse Effects:
Oral: Diarrhea, flatulence.
Cardiovascular ...In one clinical trial, 2% of patients experienced hyperlipidemia and hypertension after consumption of pomegranate juice (69175). However, most clinical research shows that pomegranate does not increase cholesterol or blood pressure and may actually improve these parameters in some patients (8310,13022,13023,69168,69373,69374).
Dermatologic ...Topically, pomegranate may cause urticaria (hives) in some patients (8445).
Gastrointestinal ...Orally, pomegranate may cause mild gastrointestinal adverse effects. In one clinical study, drinking pomegranate juice 8 ounces daily caused diarrhea and flatulence in 2% of patients (69175). In another clinical study, taking pomegranate extract (POMx, POM Wonderful LLC) 3000 mg daily caused diarrhea in 10% of patients. This dose of pomegranate extract also caused nausea, abdominal pain, constipation, gastrointestinal upset, and vomiting in a small number of patients (91695).
Immunologic
...Orally, pomegranate fruit or seeds may cause allergic reactions.
These allergic reactions occur more commonly in people who are allergic to other plants (7674). In rare cases, pomegranate fruit can cause angioedema. Angioedema seems to occur without warning and in people who have eaten pomegranate for many years. Patients should be told to stop eating pomegranate if swelling of the tongue or face develops (7673). In one report, a patient experienced pomegranate-dependent, exercise-induced anaphylaxis. The patient developed widespread urticaria (hives) and lip edema after eating pomegranate seeds and then exercising (17331). In another report, an atopic patient experienced an allergic reaction to pomegranate fruit. Symptoms included urticaria (hives), facial angioedema, and hypotension (91692).
Topically, pomegranate may cause contact hypersensitivity characterized by urticaria (hives), angioedema, rhinorrhea, red itchy eyes, and dyspnea arising within a few minutes of exposure (8445).
Pulmonary/Respiratory ...Orally, pomegranate juice may cause nasal congestion, but this event is rare. In one clinical study, pomegranate juice was associated with nasal congestion in 2% of patients (69175). There is also one case report of a 7-year-old asthmatic child who developed bronchospasm moments after ingesting several pomegranate seeds (69149).
General
...Orally, soy is well tolerated.
Most Common Adverse Effects:
Orally: Bloating, constipation, diarrhea, and nausea.
All ROAs: Allergic reactions.
Endocrine
...In the 1950s and 1960s, cases of altered thyroid function, particularly goiter, were reported in children taking soy formula.
However, adding iodine to soy formula or replacing soy flour in formula with soy protein isolate has nearly eliminated the risk of altered thyroid function in most infants (75353,75651).
In adults, there is some evidence that soy intake can alter thyroid function. Results from one clinical trial suggests that consuming soybeans 30 grams daily for as little as one month can increase thyroid-stimulating hormone (TSH) and decrease thyroxine, causing diffuse goiters, constipation, fatigue, and lethargy in some Japanese men. Recovery was achieved by discontinuing soybean intake (75206,75353). There is also some evidence that soy inhibits thyroid hormone synthesis resulting in increased secretion of TSH in some postmenopausal patients (7806). However, this seems to only occur in people with iodine deficiency (6466,75311). In postmenopausal patients with normal levels of iodine, taking a soy extract for 6 months does not seem to significantly affect thyroid hormone levels (13010).
Evidence from a single case-control study suggests that consumption of soy-based formulas may be associated with an observed three-fold increase in the risk of breast development in Puerto Rican children less than 2 years-old (75520). The correlation has been attributed to the estrogenic activity of soy. However, other risk factors, including a maternal history of ovarian cysts and consumption of meat products were also associated with the increased risk of breast development prior to 2 years of age. Also, the investigators noted that in over half of the cases, the child had not been exposed to soy or any of the other risk factors. Therefore, factors other than soy consumption may be more strongly associated with the increased risk of breast development prior to 2 years of age.
Gastrointestinal ...Gastrointestinal upset, such as constipation, diarrhea, bloating, and nausea are the most common side effects of soy (2297,11033,11082,15851,75491,95999). Reports of "bad taste" and taste intolerance have also been documented in clinical research (15851,39007,75491). Firmer stools, diarrhea, colitis, and intestinal mucosal damage has been reported in infants fed soy protein formula (75161,75448,75516,75525).
Genitourinary
...Orally, soy might increase discomfort during menstrual periods.
Evidence from a small, retrospective cohort study has found that consuming soy formula as an infant may slightly increase the duration and discomfort of menstrual periods later in life. However, the investigators noted that these differences may not be clinically significant (7331).
Orally, frequent soy consumption might be a risk factor for uterine leiomyoma, an estrogen-dependent benign tumor located on the uterus. Observational research found that consumption of soy milk or soybean at least four times weekly is associated with a 7-fold increased odds of uterine leiomyoma (98869).
There is some concern that use of soy-based formulas in infants might result in long-term health complications. However, results from a retrospective cohort study has found that intake of soy-based formula as an infant does not affect height, weight, body mass index, pubertal maturation, menstrual history, or pregnancy history, nor does it increase the risk of reproductive organ disorders, hormonal disorders, libido dysfunction, or birth defects in the offspring of adults who received soy formula as infants (7331,11080). Additionally, research in adults shows that urinary phytoestrogens are not associated with endometriosis risk (101804). However, some population research has found that regular exposure to soy-based formulas during infancy is associated with an increased risk for endometriosis (101803).
Immunologic
...Orally, soy can cause allergic reactions such as skin rash and itching in some people (6412).
In an 11-year-old female, allergy to soy protein resulting in a delayed itching papular rash was thought to be responsible for the reaction to injected benzathine benzylpenicillin containing possible soy protein-contaminated soy lecithin (96422).
Topically, soy-based ingredients were responsible for the development of hand atopic dermatitis in a young female using cosmetic lotions in the workplace. Percutaneous sensitization resulted in the development of anaphylaxis to oral soy (96000).
Neurologic/CNS ...Orally, one clinical study showed that insomnia was more common in postmenopausal adults taking soy isoflavone supplements when compared with those receiving placebo (9917). Some research suggests that dietary consumption of tofu during midlife might decrease cognitive function in later years. Evidence from one retrospective cohort study suggests that males who consume at least two servings of tofu weekly during midlife have increased risk of cognitive impairment in late life (19% vs. 4%) compared to those who consume tofu less frequently. Although the effect of tofu was considered to be marginal compared to other factors such as age, education, or history of stroke, results from the study suggest that the effect of significant midlife consumption of tofu is comparable to the effect of an age difference of 4 years or an education difference of 3 years. However, numerous other factors, such as lifestyle and health, could be involved (6415,6416). Therefore, these findings are too preliminary to be used as a basis for clinical recommendations.
Oncologic
...There is controversy about the role of soy in breast cancer.
Population studies suggest that soy is protective against breast cancer. Asian females who eat a traditional diet high in soy seem to have a lower risk of developing breast cancer (4590,5939,9674). Early exploratory studies have suggested that soy stimulates proliferation of normal human breast tissue (3980,3981). However, taking a soy tablet containing 50 mg soy isoflavones daily for 12 months does not alter mammographic or breast MRI tissue density in adults at high risk of breast cancer, with non-endocrine treated breast cancer, or previously treated for breast cancer and without evidence of recurrence (95999).
There is some concern that soy supplements, but not soy foods, might increase the risk of endometrial hyperplasia due to its estrogenic effects. Population and clinical research suggests that soy foods do not have a proliferative effect on endometrial cells (7358,2429,7654,9676,9917), and increased dietary soy and phytoestrogens are associated with reduced endometrial cancer risk (7338,10372). However, the effects seem to be different with concentrated soy isoflavone extract. While taking products providing isoflavones 120 mg daily for 6 months does not increase endometrial thickening (13209), taking higher doses such as isoflavones 150 mg daily for 5 years might increase the risk of simple endometrial hyperplasia (12105). However, there is no evidence that soy isoflavones increase the risk of atypical hyperplasia which has a much higher risk of developing into endometrial cancer than simple endometrial hyperplasia (12105,90973).
There is also concern that increased soy intake increases the risk for other types of cancer. Some observational research has found that higher dietary intake of soy is associated with a higher risk for bladder cancer and pancreatic cancer (9677,105609).
A meta-analysis of results from cohort and case-control studies evaluating the risk of stomach cancer related to consumption of fermented soy products is unclear and inconclusive. The highest quality data from cohort studies suggests that these products have no significant effect on stomach cancer (7340,7341). More research is required to determine if soy products have any correlation with stomach cancer.
Pulmonary/Respiratory ...Inhaled soy dust and soy hull aeroallergen can trigger symptoms of asthma and allergic rhinitis (5084,5085,5086).
