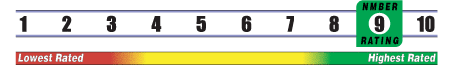
| Ingredients | Amount Per Serving |
|---|---|
|
(reduced CoQ10)
|
50 mg |
Medium Chain Triglyceride (Alt. Name: MCT), Food Starch, Glycerin, Carrageenan, Water, Purified, Lycopene Complex, White Beeswax, Sunflower Lecithin, Ascorbyl Palmitate
Below is general information about the effectiveness of the known ingredients contained in the product CellularActive CoQ10 Ubiquinol 50 mg. Some ingredients may not be listed. This information does NOT represent a recommendation for or a test of this specific product as a whole.
INSUFFICIENT RELIABLE EVIDENCE to RATE
Below is general information about the safety of the known ingredients contained in the product CellularActive CoQ10 Ubiquinol 50 mg. Some ingredients may not be listed. This information does NOT represent a recommendation for or a test of this specific product as a whole.
LIKELY SAFE ...when used orally and appropriately. Coenzyme Q10 has been used safely in studies lasting up to 5 years (2134,6037,6038,6407,8163,8938,8939,8940,15395,17413,17716,96538)(109391). ...when used topically on the gums (2107,2108,8916,8917,8918).
CHILDREN: POSSIBLY SAFE
when used orally and appropriately.
Coenzyme Q10 in doses of 1-10 mg/kg/day has been used safely for up to 9 months under medical supervision (12199,13223,15256,44005,107449).
PREGNANCY: POSSIBLY SAFE
when used orally and appropriately.
Coenzyme Q10 100 mg twice daily has been used with apparent safety during pregnancy, starting at 20 weeks gestation until term (17201).
LACTATION:
Insufficient reliable information available; avoid using.
Below is general information about the interactions of the known ingredients contained in the product CellularActive CoQ10 Ubiquinol 50 mg. Some ingredients may not be listed. This information does NOT represent a recommendation for or a test of this specific product as a whole.
Coenzyme Q10 has antioxidant effects. Theoretically, this may reduce the activity of chemotherapy drugs that generate free radicals.
|
Theoretically, coenzyme Q10 might have additive effects with antihypertensive drugs.
|
Coenzyme Q10 is chemically similar to menaquinone and might have vitamin K-like procoagulant effects, which could decrease the effects of warfarin.
Concomitant use of coenzyme Q10 and warfarin might reduce the anticoagulant effects of warfarin (2128,6048,6199). Four cases of decreased warfarin efficacy thought to be due to coenzyme Q10 have been reported (2128,6048,11048). However, there is some preliminary clinical research that suggests coenzyme Q10 might not significantly decrease the effects of warfarin in patients who have a stable INR (11905).
|
Below is general information about the adverse effects of the known ingredients contained in the product CellularActive CoQ10 Ubiquinol 50 mg. Some ingredients may not be listed. This information does NOT represent a recommendation for or a test of this specific product as a whole.
General
...Orally, coenzyme Q10 is generally well tolerated.
In clinical studies, no serious adverse effects have been reported.
Most Common Adverse Effects:
Orally: Gastrointestinal side effects such as appetite suppression, diarrhea, epigastric discomfort, heartburn, nausea, and vomiting. These generally occur in less than 1% of patients. Some of these adverse effects can be minimized if daily doses above 100 mg are divided.
Cardiovascular ...Palpitations have been reported as being possibly associated with coenzyme Q10 treatment (89421). Death due to myocardial infarction occurred in one Parkinson disease patient taking coenzyme Q10; causality is unclear (15395).
Dermatologic ...Two of 143 participants in a case series reported skin itching after starting treatment with oral coenzyme Q10 (6047). Allergic rash has also been reported (6409,11872). An itching exanthema was seen in two heart failure patients treated with intravenous coenzyme Q10 (44284).
Gastrointestinal ...Gastrointestinal side effects of coenzyme Q10 have included nausea (3365,6409,8907,10152,43982,44172,44179,44330,89421,109392), vomiting (3365,10152,44330,89421), epigastric discomfort (3365,44179,44330,89421), constipation (109392), diarrhea (44179,92904,89421,109392), stomach upset (8940,12170,109387,109388,109392), loss of appetite (2121), heartburn (2121,44179,109392), and flatulence (43982), although this occurs in less than 1% of patients. In one clinical study, gastrointestinal bleeding in association with angiodysplasia has been reported to be possibly related to coenzyme Q10 treatment (89421).
Genitourinary ...An uncomplicated urinary infection was reported in a patient taking oral coenzyme Q10 (nanoQuinon, MSE Pharmazeutika) (44020).
Hematologic ...Thrombocytopenia was noted in one patient treated with oral coenzyme Q10 (44296); however, other factors (viral infection, other medications) may have been responsible for this adverse effect.
Musculoskeletal ...Increased plasma creatine kinase with high-intensity exercise has been reported in patients taking coenzyme Q10 (44303). Muscle pain has been reported rarely in one clinical trial (109392).
Neurologic/CNS ...Headache and dizziness have been reported in human research (3365,11872,43982,44330,109392). Insomnia has been reported as being possibly associated with coenzyme Q10 treatment (89421). Cognitive decline, depression, and sudden falls were reported rarely in a clinical trial of patients with Huntington disease (8940). Increased lethargy was reported for one patient treated with oral coenzyme Q10 (44042). Feeling of internal trembling has been reported in a clinical trial for one patient treated with coenzyme Q10 (44020).
Ocular/Otic
...Visual sensitivity to light has been reported for a patient treated with coenzyme Q10.
However, the association of this effect with coenzyme Q10 treatment was not clear (6409).
A burning sensation has been reported for 10% of patients treated with a topical eye solution containing coenzyme Q10 and alpha-tocopheryl polyethylene glycol 1000 succinate following cataract surgery (44228).
Psychiatric ...Worsening depression has been reported as being possibly associated with oral coenzyme Q10 treatment (89421).
Pulmonary/Respiratory ...Drug-induced pneumonitis was diagnosed in a 61 year-old woman who had been taking coenzyme Q10 and perilla leaf extract for two months (43978). Symptoms improved after she stopped taking the supplements and began taking oral prednisone. Causation from coenzyme Q10 was unclear.
Other ...In a case report, a naval aviator using a supplement containing coenzyme Q10 and niacin had reduced G tolerance (44186). G tolerance was regained with cessation of the supplement.
