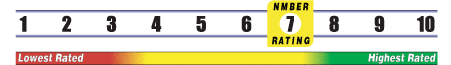
| Ingredients | Amount Per Serving |
|---|---|
|
(Red Cinchona Bark Glycerite Liquid Extract)
(Red Cinchona Bark Glycerite Liquid Extract 1:5)
|
0.13 mL |
Glycerin, Chocolate flavor
Below is general information about the effectiveness of the known ingredients contained in the product Quinine Bark (Red Cinchona) Chocolate Flavor. Some ingredients may not be listed. This information does NOT represent a recommendation for or a test of this specific product as a whole.
INSUFFICIENT RELIABLE EVIDENCE to RATE
Below is general information about the safety of the known ingredients contained in the product Quinine Bark (Red Cinchona) Chocolate Flavor. Some ingredients may not be listed. This information does NOT represent a recommendation for or a test of this specific product as a whole.
LIKELY SAFE ...when used orally as a flavoring in tonic water and alcoholic beverages. The US Code of Federal Regulations allows not more than 83 parts per million (ppm) of total cinchona alkaloids in finished beverages (93229).
POSSIBLY UNSAFE ...when used orally in medicinal amounts. Cinchona derivatives marketed as over-the-counter (OTC) medicines are required to carry the warning, "Caution - discontinue use if ringing in the ears, deafness, skin rash, or visual disturbances occur" (93231). Cinchona contains the alkaloid quinine that was previously available OTC in the US for treatment and prevention of nocturnal leg muscle cramps. In 1994 the US Food and Drug Administration (FDA) determined that quinine was not generally recognized as safe and effective for this indication, citing serious adverse reactions and its narrow therapeutic index (93232,93233). A final ban on marketing of OTC quinine products was implemented by the FDA in 2007, and a Risk Evaluation and Mitigation Strategy (REMS) to reduce off-label use of prescription quinine products for night-time leg cramps was introduced in 2010 (93232).
LIKELY UNSAFE ...when excessive amounts are used orally. Cinchona contains the alkaloids quinine and quinidine, which are used as prescription medicines and have been associated with significant adverse effects at doses of 2 grams per day or more (505). The amount of these constituents in cinchona products is variable (13).
PREGNANCY: LIKELY UNSAFE
when used orally.
Cinchona is reported to have uterine stimulant and abortifacient activity, and to be fetotoxic and teratogenic, causing visual and auditory defects (12,19). Avoid using.
LACTATION: POSSIBLY UNSAFE
when used orally.
The cinchona alkaloids quinine and quinidine are reported to be excreted in breast milk and may be toxic to infants (19).
Below is general information about the interactions of the known ingredients contained in the product Quinine Bark (Red Cinchona) Chocolate Flavor. Some ingredients may not be listed. This information does NOT represent a recommendation for or a test of this specific product as a whole.
Theoretically, taking cinchona might decrease the effectiveness of antacids. Theoretically, taking antacids might also increase the risk of adverse effects from cinchona.
Some research shows that taking cinchona lowers stomach acid pH (19). In addition, some research shows that taking antacids might increase urinary pH. Theoretically, this may increase the amount of quinidine, a constituent of cinchona, reabsorbed in the renal tubules and increase the risk of quinidine toxicity (3046).
|
Theoretically, taking cinchona might increase the drug effects and risk of bleeding with anticoagulant and antiplatelet drugs.
|
Theoretically, taking cinchona might increase the adverse effects of carbamazepine.
Clinical research shows that taking quinine, a constituent of cinchona, increases the peak plasma concentration and area under the curve of carbamazepine (11016).
|
Theoretically, taking cinchona might inhibit cytochrome P450 2D6 (CYP2D6) and increase levels of drugs metabolized by this enzyme.
|
Theoretically, taking cinchona might increase serum levels of digoxin.
Quinine and quinidine, which are constituents of cinchona, decrease clearance of digoxin and increase serum digoxin levels in humans (3046).
|
Theoretically, taking cinchona might decrease the effectiveness of H2-blockers.
Some research shows that taking cinchona lowers stomach acid pH (19).
|
Theoretically, taking cinchona might increase the adverse effects of phenobarbital.
|
Theoretically, taking cinchona might decrease the effectiveness of PPIs.
Some research shows that taking cinchona lowers stomach acid pH (19).
|
Theoretically, taking cinchona with other QT interval-prolonging drugs might cause an additive effect and increase the risk of ventricular arrhythmias.
Quinidine and quinine, which are constituents of cinchona, prolong the QT interval (3046).
|
Theoretically, taking cinchona might increase plasma levels and adverse effects of quinidine.
Cinchona contains quinidine (505).
|
Theoretically, taking cinchona might increase plasma levels and adverse effects of quinine.
Cinchona contains quinine (505).
|
Below is general information about the adverse effects of the known ingredients contained in the product Quinine Bark (Red Cinchona) Chocolate Flavor. Some ingredients may not be listed. This information does NOT represent a recommendation for or a test of this specific product as a whole.
General
...Information on the adverse effects of cinchona is limited.
Orally, prolonged use of high doses of cinchona can cause severe adverse effects. Topically, a thorough evaluation of safety outcomes has not been conducted.
Most Common Adverse Effects:
Orally: Diarrhea, nausea, ringing ears, vomiting.
Topically: Contact dermatitis, urticaria.
Serious Adverse Effects (Rare):
Orally: Arrhythmias, cinchonism syndrome, hemolytic uremic syndrome, QT prolongation, thrombotic thrombocytopenic purpura.
Cardiovascular ...Cinchona contains the alkaloids quinidine and quinine that can prolong the QT interval on the electrocardiogram, and cause potentially fatal cardiac arrhythmias such as torsades de pointes (3046,93232)
Dermatologic ...Topical use of cinchona bark extracts and occupational exposure to cinchona bark dust can cause contact dermatitis and other urticarial reactions (11,93234). A 31-year old man developed itching, erythema, and edema of the face and upper chest after occupational exposure to dust from cinchona bark. Skin testing produced reactions to ethanol and ether extracts of the bark, but not to the individual alkaloids quinine and quinidine (93234).
Gastrointestinal ...Cinchona stimulates secretion of stomach acid and has been associated with nausea, vomiting, and diarrhea (6,19).
Hematologic ...Quinine, which is present in cinchona, has been associated with serious, sometimes fatal, hematological disorders including thrombocytopenia, thrombotic thrombocytopenic purpura (TTP), and hemolytic uremic syndrome (hemolytic anemia, acute renal failure, and thrombocytopenia) (93232,93233). Initial symptoms may include bleeding from the gums, nose or gastrointestinal tract, easy bruising, and petechiae (93233). Bone marrow depression and thrombocytopenia have also been associated with quinidine (505).
Immunologic ...Oral use of quinine, an alkaloid present in cinchona, has been associated with severe allergic skin reactions, as well as anaphylaxis (19,93232).
Ocular/Otic ...Cinchona contains quinine that can cause dose-related adverse effects on hearing and vision, including tinnitus, deafness, vision changes, and blindness (6,8,12,93232).
Other ...Orally, prolonged use of high doses of cinchona or its alkaloids, or a single dose of 3 grams or more of the alkaloid quinine are associated with a toxicity syndrome known as cinchonism. Symptoms include headache, dizziness, nausea and vomiting, diarrhea, hemolysis, hypotension, cardiac arrhythmias, tinnitus, deafness, vision changes, blindness, abdominal pain, delirium, convulsions, paralysis, and collapse (6,12,19,505,93232). Doses of 10-15 grams of quinine may be fatal (18).
