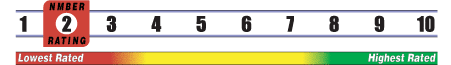
Two capsules contain: pharmaceutical grade Androstenedione (Delta-4-androstene-3-17-dione) 100 mg • pharmaceutical grade Dehydroepiandrosterone (Dehydroepiandrosterone) 100 mg • Tribulus terrestris 650 mg.
Brand name products often contain multiple ingredients. To read detailed information about each ingredient, click on the link for the individual ingredient shown above.
This product has been discontinued by the manufacturer.This product contains the ingredient androstenedione. In March 2004, the U.S. Food and Drug Administration told manufacturers of products containing this ingredient to immediately stop distribution. The FDA considers these dietary supplements to be adulterated drugs due the lack of historical data regarding dietary use that establishes a reasonable expectation of safety. There are concerns that these products are unsafe. Androstenedione might have potent androgenic effects and lead to serious side effects including impotence, liver problems, and increased cancer risk (12001). In January 2005 legislation went into effect in the United States called the Anabolic Steroid Control Act of 2004. This reclassifies androstenedione from a dietary supplement to an anabolic steroid, which is a schedule III controlled substance (8639).
Below is general information about the effectiveness of the known ingredients contained in the product Andro-Stack 850. Some ingredients may not be listed. This information does NOT represent a recommendation for or a test of this specific product as a whole.
INSUFFICIENT RELIABLE EVIDENCE to RATE
INSUFFICIENT RELIABLE EVIDENCE to RATE
INSUFFICIENT RELIABLE EVIDENCE to RATE
Below is general information about the safety of the known ingredients contained in the product Andro-Stack 850. Some ingredients may not be listed. This information does NOT represent a recommendation for or a test of this specific product as a whole.
POSSIBLY UNSAFE ...when used orally. Androstenedione has been associated with significant side effects, including increased risk of breast, pancreatic, and prostate cancer (672,3861,6000). In addition, there are safety concerns that the potency and purity of androstenedione products can differ significantly from the product labeling (10641).
CHILDREN: LIKELY UNSAFE
when used orally.
Androstenedione could potentially cause premature closure of the bone growth plates and precocious puberty (674,111132).
PREGNANCY: LIKELY UNSAFE
when used orally.
Androstenedione might induce labor (673).
LACTATION:
Insufficient reliable information available; avoid using.
POSSIBLY SAFE ...when used orally and appropriately, short-term. Most studies have been small and lasted from a few weeks to 6 months, with usual doses of 50 mg daily (793,1635,2133,3231,4249,4251,4252,4253,4254,4255,9691)(9692,10986,12215,12564,14277,21416,88726,90304,99925). Some studies have also used oral DHEA with apparent safety for 12-24 months (2113,6446,10406,11464,12561,15027,88492). ...when used intravaginally and appropriately. Intravaginal ovules of DHEA 3.25 mg to 13 mg have been safely used for up to 12 weeks (21320,21429,21430). ...when used topically and appropriately. A DHEA cream 1% to 10% has been safely used for up to 12 months (4242,21428).
POSSIBLY UNSAFE ...when used orally in high doses or long-term. There is concern that long-term use or use of amounts that cause higher than normal physiological DHEA levels might increase the risk of prostate cancer (2111,12565), breast cancer (10370,10401,10403), or other hormone-sensitive cancers (6445). In some cases, 50-100 mg daily can produce slightly higher than normal physiological DHEA levels (4249,4251). There is insufficient reliable information available about the safety of using DHEA intravenously or intramuscularly.
PREGNANCY AND LACTATION: POSSIBLY UNSAFE
when used orally.
DHEA can cause higher than normal androgen levels (2133,4249,4251,4253), which might adversely affect pregnancy or a nursing infant.
LIKELY UNSAFE ...when the spine-covered fruit is used orally. There have been reports of bilateral pneumothorax and bronchial polyp after oral consumption of the spine-covered fruit (818).
PREGNANCY: POSSIBLY UNSAFE
when used orally.
Animal research suggests that tribulus might adversely affect fetal development (12674); avoid using.
LACTATION:
Insufficient reliable information available; avoid using.
Below is general information about the interactions of the known ingredients contained in the product Andro-Stack 850. Some ingredients may not be listed. This information does NOT represent a recommendation for or a test of this specific product as a whole.
Theoretically, androstenedione might increase the effects and adverse effects of estrogens.
Androstenedione is a precursor to estrogen and seems to increase estrogen levels (1905,3861,6000,7236,8138,19563,97845,111132,111134). However, androstenedione is converted to estrogen only in low estrogen environments. Patients taking estrogen do not have low estrogen levels because of the estrogen treatment. Therefore, androstenedione is unlikely to be converted to estrogen in these patients. However, until more is known, use with caution or avoid using.
|
Theoretically, DHEA might increase the risk of bleeding if used with anticoagulant or antiplatelet drugs.
|
Theoretically, DHEA might increase the risk of psychiatric adverse events when used with antidepressants.
In a human case report, the use of a selective serotonin reuptake inhibitor (SSRI) with DHEA caused a manic episode (7023). Concern for this interaction may be greater in younger individuals with higher baseline DHEA levels.
|
Theoretically, DHEA might interfere with the clinical effects of aromatase inhibitors.
DHEA is a potent estrogen agonist, which may antagonize the anti-estrogen activity of aromatase inhibitors (10401).
|
Theoretically, DHEA might increase the levels of drugs metabolized by CYP3A4.
Some preliminary evidence shows that DHEA may inhibit CYP3A4 (1389); however, the clinical significance of this potential interaction is not known.
|
Theoretically, DHEA might increase the effects and adverse effects of estrogen therapy.
DHEA is a precursor to estrogen and androgen and is metabolized into those substances. In clinical research, DHEA supplements increase the levels of these hormones (6012,7614,8593,10986,12651,12564,15027,21321,21323,21324)(21325,21326,21327,21328,21330,21331,21356,21364,21389,21393)(21397,21398,21417,21419,21427,47273,47348,88375,90304). Also, in clinical research, estrogen-progestin oral contraceptives and conjugated estrogens reduce blood levels of DHEA and DHEA-S (21372,21373,21374,21437,21438). The clinical significance of these findings is unclear.
|
Theoretically, DHEA might interfere with the anti-estrogen effects of fulvestrant.
|
Theoretically, DHEA might interfere with the anti-estrogen effects of tamoxifen.
|
Theoretically, DHEA might increase the effects and side effects of testosterone therapy.
DHEA is a precursor to estrogen and androgen and is metabolized into those substances. In clinical research, DHEA supplements increase the levels of these hormones (6012,7614,8593,10986,12651,12564,15027,21321,21323,21324)(21325,21326,21327,21328,21330,21331,21356,21364,21389,21393)(21397,21398,21417,21419,21427,47273,47348,88375,90304,99924,99925,104162). The clinical significance of these findings is unclear.
|
DHEA can increase blood levels of triazolam.
Administration of DHEA 200 mg daily for two weeks was shown to inhibit the cytochrome P450 3A4 (CYP3A4) metabolism of triazolam. This inhibition appears to be due to DHEA-S, rather than DHEA (1389).
|
DHEA might reduce the effectiveness of the tuberculosis vaccine.
Animal research shows that high doses of DHEA can reduce the efficacy of the Bacillus Calmette-Guérin (BCG) tuberculosis vaccine (21316).
|
Taking tribulus with antidiabetes drugs might increase the risk of hypoglycemia.
Clinical research shows that Tribulus can lower blood glucose levels in adults with type 2 diabetes who are taking antidiabetes medications (97327).
|
Theoretically, taking tribulus with antihypertensive drugs might increase the risk of hypotension.
|
Theoretically, tribulus might increase the levels and clinical effects of lithium.
Tribulus is thought to have diuretic properties (12681). Due to these potential diuretic effects, tribulus might reduce excretion and increase levels of lithium. The dose of lithium might need to be decreased.
|
Below is general information about the adverse effects of the known ingredients contained in the product Andro-Stack 850. Some ingredients may not be listed. This information does NOT represent a recommendation for or a test of this specific product as a whole.
General
...Orally, androstenedione may be unsafe.
Serious Adverse Effects (Rare):
Orally: hepatotoxicity; breast and endometrial cancer (females); pancreatic and prostate cancer (males); and adverse genitourinary effects.
Cardiovascular ...Orally, there is some concern that androstenedione might increase risk of heart disease in men. Androstenedione decreases high-density lipoprotein (HDL) cholesterol (1905,6000,7236,31279,111134).
Dermatologic ...Orally, androstenedione might cause acne in men by decreasing endogenous testosterone production and increasing estrogen. In women, androstenedione might cause male-pattern baldness, hair growth, acne, and coarsening of the skin (674,111132).
Endocrine ...Orally, androstenedione might decrease endogenous testosterone production and increase estrogen in men (1905,3861,6000,7236,8138,97845). Changes in testosterone levels are variable and might depend on length of supplementation and age (1905,3861,6000,7236,8138,31250,97845,111132). In women, androstenedione can increase testosterone levels (19563,19564,111132). Theoretically, these changes can affect behavior and physical characteristics.
Genitourinary ...Orally, androstenedione possibly alters levels of estrogen and testosterone in men (1905,3861,6000,7236,8138,97845,111132,111134). Theoretically, this might cause decreased spermatogenesis, gynecomastia, testicular atrophy, and prostate hypertrophy (6000,31266,111132). There is one case report of two episodes of priapism associated with androstenedione use in a 30-year-old man (5089). Also, there is one case report of severe oligospermia, reduced libido, impotence, and testicular atrophy in a 29-year-old male recreational bodybuilder using androstenedione (97844). In women, androstenedione may theoretically cause clitoral hypertrophy and menorrhea (674,31266,31280,111132).
Hepatic ...Orally, androstenedione might cause hepatotoxicity. Testosterone derivatives similar to androstenedione have been associated with hepatic toxicity (3861). Consider monitoring liver function tests (LFTs) in patients using androstenedione.
Oncologic ...Orally, androstenedione might potentially increase the risk of pancreatic and prostate cancer in men (672,6000). There is preliminary evidence that androstenedione might stimulate prostate cancer cell growth (672,8138,31280,31283). However, population research shows that higher blood levels of androstenedione is not associated with an increased risk of prostate cancer in mean with enlarged prostates (97841). Some androstenedione products are combined with other supplements such as dehydroepiandrosterone, puncture vine, saw palmetto, indole-3-carbinol, and chrysin (DION) to lessen the effects of androstenedione on increased levels of estradiol and DHT. However, this combination does not seem to help (8138). In women, androstenedione can increase testosterone levels. Theoretically, this might increase the risk of breast and endometrial cancer (31273).
Psychiatric ...There is some concern that androstenedione might cause or worsen depression in women. Some women with severe major depression seem to have increased endogenous androstenedione concentrations; however, it is not known if supplements can actually cause this adverse effect (6796).
General
...Orally and topically, DHEA seems to be well tolerated when used in typical doses, short-term.
However, there is some concern that long-term oral use of DHEA may be linked to a greater risk for cancer.
Most Common Adverse Effects:
Orally: Acne, headache, insomnia, mood changes, and nausea. In females, masculinization symptoms including deepening of the voice, increased size of genitals, irregular menses, oily skin, reduced breast size, and unnatural hair growth. In males, aggression, breast tenderness or enlargement (gynecomastia), urinary urgency, and testicular wasting.
Serious Adverse Effects (Rare):
Orally: Possible increased risk for cardiovascular events and various types of cancer.
Cardiovascular ...Incidences of arrhythmia (21334,47540), chest pain (21332,21333), palpitations (21332,21333,88492), hypertension, and transient ischemic attacks (21353,21354,47300) have been reported. DHEA has also been found to decrease high-density lipoprotein (HDL) levels (21344,21345,21346,21347,21348,21349) and increase triglycerides (21334).
Dermatologic ...Acne has been the most commonly reported adverse effect in human research, particularly in females (2113,2114,4242,7614,7559,12561,12574,21346,21351,21354)(21355,21356,21357,21358,21360,21361,21362,21363,21364,47300)(47355,47409,90304,103185). However, it is generally mild and may be treated by reducing the dose (7559). Incidences of contact dermatitis (47402), acneiform dermatitis (2113), greasy hair and skin (17218,21351,21355,21363,21387,21389,47355), keratosis (47402), skin rash (12574,21361,21363), erythema (21334), scalp itching (17218,21357), and skin spots (21387) have also been reported. Increased hair growth and hirsutism have been noted in several clinical trials, including the development of mild mustache in females (2114,4242,12561,12574,17218,21346,21351,21354,21355,21358) (21361,21362,21363,21370,21387,21389,21415,47300). Increased perspiration and odor have also been reported in human research (17218,21354,21356,21357).
Endocrine ...In postmenopausal patients, high doses of DHEA (1600 mg daily) induced insulin resistance, reportedly due to increased androgen levels that occurred during supplementation (21324).
Gastrointestinal ...Gastrointestinal disturbances such as nausea, diarrhea, and abdominal discomfort have been noted in human research (2111,6098,7559,12574,21348,21358,21386).
Genitourinary ...In older adults, elevated and severe urinary symptoms (as evidenced by scores of more than 20, using the American Urological Association Symptom Index for Benign Prostatic Hyperplasia [International Prostate Symptom Score]) and urinary tract infection were reported (21353). Rare incidences of abnormal menses (2114) and increased discharge (21415) have been reported. DHEA has been associated with hematuria (47300).
Hepatic ...Elevated liver enzymes have been reported following DHEA supplementation (21364,47300). However, an analysis of multiple studies in varied patient populations taking DHEA supplements found no elevations in liver enzymes (107791).
Musculoskeletal ...Incidences of asthenia, arthralgia, and myalgia, including calf cramps, have been reported (12574,21354,21358,21365,47355).
Neurologic/CNS ...In humans, dizziness, fatigue, malaise, sleep disturbances, increased dreaming, night sweats, restlessness, "painful spots," and a crawling scalp sensation have been reported (3865,21354,21363,21389). There is a case of seizure associated with DHEA use in a 30 year-old female with fragile X syndrome and no history of convulsive disorder who used DHEA to try to improve ovarian production (47344).
Ocular/Otic ...In patients with Sjögren syndrome, maculae lesions, ocular pain and dryness, and painful eye exams have been reported (21358,21363,21365).
Oncologic ...Preclinical research suggests that DHEA may increase the risk of cancer, particularly prostate, liver, breast, and pancreatic cancers (2111,10370,10401,10403,12565,21332,21333,21334,47251,47256)(47366,47388,47539). High concentrations of DHEA in postmenopausal patients have been associated with an increased risk of breast cancer (2115,6445).
Psychiatric ...DHEA-induced mania has been reported (5870,6102,7023,21383). Clinical studies have also reported anxiety, nervousness, irritability, emotional change, and depression in patients receiving DHEA (2114,21358,21360,21370).
Pulmonary/Respiratory ...Increased cough and nasal congestion have been noted in human research (3865,11334). A report of acute respiratory failure was made in clinical study evaluating the use of DHEA in patients with myotonic dystrophy (type 1) (21334).
Other ...Perceived increases in weight gain have been reported with use of DHEA (2114,21361).
General
...Orally, tribulus seems to be well tolerated.
Serious Adverse Effects (Rare):
Orally: Cases of liver and kidney injury, seizures, and chronic painful erection with impaired sexual function have been reported. Pneumothorax and bronchial polyp after consuming the spine-covered tribulus fruit have been reported.
Gastrointestinal ...Orally, tribulus can cause abdominal pain, cramping, nausea, vomiting, diarrhea, and constipation (92022,92027). However, in one study, the rates of these gastrointestinal complaints were similar for patients taking tribulus and those receiving placebo (92022).
Genitourinary ...In one case report, a patient taking two tribulus tablets (unknown dose) daily for 15 days presented to the local emergency department with a painful erection lasting 72 hours. The priapism was resolved with medical management; however, post-episode sexual function was impaired (92023).
Hepatic ...In one case report, a patient drinking tribulus water 2 liters daily for two days presented with lower limb weakness, seizures, hepatitis, and acute kidney injury. The patient's condition improved after hemodialysis and discontinuation of tribulus water (92069).
Neurologic/CNS ...Orally, tribulus has been reported to cause general excitation and insomnia. These symptoms were reversed upon discontinuation of the drug or decreasing the dose (78867). In one case report, a patient drinking tribulus water 2 liters daily for two days presented with lower limb weakness, seizures, hepatitis, and acute kidney injury. The patient's condition improved after hemodialysis and discontinuation of tribulus water (92069).
Pulmonary/Respiratory ...In one case report, a patient developed a bilateral pneumothorax after consuming the spine-covered fruit of tribulus (818). In another case report, a patient developed a polyp in the lobar bronchus of the right interior lobe due to the presence of a tribulus fruit spine (78852).
Renal ...In one case report, a patient drinking tribulus water 2 liters daily for two days presented with lower limb weakness, seizures, hepatitis, and acute kidney injury. The patient's condition improved after hemodialysis and discontinuation of the tribulus water (92069). In another case report, a healthy male taking one tribulus tablet (unknown dose) daily for a few months for bodybuilding purposes developed hyperbilirubinemia followed by acute kidney failure 2-3 weeks later. The patient was managed with intravenous fluids and a low-salt, low-protein diet (92025).
Other ...In one case report, gynecomastia was observed in a male weightlifter taking an herbal combination product containing tribulus. However, it is not clear if this adverse effect can be attributed to tribulus alone (78859).
