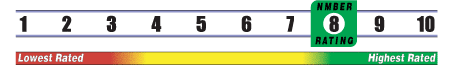
| Ingredients | Amount Per Serving |
|---|---|
|
( C22:6n-3, DHA)
(Algal Oil)
|
200 mg |
Water, soluble Food Starch, Glycerin, Carrageenan, Sorbitol, Caramel, Beta-Carotene, Sunflower Oil, Tocopherol, Natural Flavors, Sunflower Lecithin, Ascorbyl Palmitate
Below is general information about the effectiveness of the known ingredients contained in the product Omega-3 Vegetarian DHA 200 mg. Some ingredients may not be listed. This information does NOT represent a recommendation for or a test of this specific product as a whole.
INSUFFICIENT RELIABLE EVIDENCE to RATE
Below is general information about the safety of the known ingredients contained in the product Omega-3 Vegetarian DHA 200 mg. Some ingredients may not be listed. This information does NOT represent a recommendation for or a test of this specific product as a whole.
LIKELY SAFE ...when used orally and appropriately. DHA has been used safely in studies lasting up to 4 years (1016,1043,6413,10321,10869,11333,90684). Fish oil supplements containing DHA have also been safely used in studies lasting up to 7 years (1016). While doses of DHA up to 4 grams orally daily have been used safely in some clinical research (6143), there is some concern that high intake of omega-3 fatty acids such as DHA might increase the risk of bleeding. For this reason, the US Food and Drug Administration (FDA) recommends that consumers limit intake of DHA plus eicosapentaenoic acid (EPA), another omega-3 fatty acid also found in fish oil, to 3 grams daily, with no more than 2 grams daily from a dietary supplement (95739).
POSSIBLY SAFE ...when used intravenously and appropriately, in combination with eicosapentaenoic acid (EPA), short-term. Daily infusions with an omega-3 fatty acid-based lipid emulsion (Omegavenous 10%, Fresenius Aktiengesellschaft) providing 4.2 grams/day of DHA and EPA has been used safely for 14 days (1004).
POSSIBLY UNSAFE ...when used orally in high doses. Doses greater than 3 grams daily might decrease platelet aggregation and increase the risk of bleeding (1313). The US Food and Drug Administration (FDA) recommends that consumers limit intake of DHA plus eicosapentaenoic acid (EPA), another omega-3 fatty acid, to 3 grams daily, with no more than 2 grams daily from a dietary supplement (95739).
CHILDREN: LIKELY SAFE
when used orally and appropriately.
DHA is a component of some infant formula (424,1045,5708,5941,7599,14403,15003,15495,17735,48088)(48194,48266,48343,90665,90713,90716,110357). In children 7 years and older, DHA 30 mg/kg daily has been used safely for up to 4 years (90684). Also, DHA 0.4-1 grams daily has been safely used in children ages 4 years and older for up to 1 year (11333,90665,100940,104560).
CHILDREN: POSSIBLY UNSAFE
when used orally in preterm infants born less than 29 weeks gestation.
Although not all findings agree (110356,110359), supplementation with an enteral emulsion containing DHA 40 mg/kg to 60 mg/kg daily might increase the risk of developing or worsening bronchopulmonary dysplasia compared to control emulsion (96523,110359).
PREGNANCY AND LACTATION: LIKELY SAFE
when used orally and appropriately.
An intake of DHA 650 mg daily from food and/or supplements during pregnancy seems to be required to prevent a reduction in DHA status before delivery (110329). DHA is commonly used during pregnancy and lactation and is a component of some prenatal supplements. DHA is a normal component of breast milk, with higher levels in breast milk following term vs. preterm pregnancies (14393,14394,14396,14400,14403,14397,20000,47977,47994,48095)(90672,90718,110355). When taken as a prenatal supplement, DHA increases DHA levels in breast milk (90685). Doses of DHA ranging from 300-600 mg daily beginning during the first trimester of pregnancy have been used safely in clinical research (90672,90676,90687,90694). When taken during lactation, DHA increases DHA levels in breast milk (109214,110362). When initiated within 72 hours of delivery of a very preterm infant, taking DHA 1.2 grams daily increases DHA levels in breast milk within 14 days (109214). One study found that DHA supplementation during lactation increased the risk of bronchopulmonary dysplasia in breast-feeding infants born less than 29 weeks gestational age (104559); however, it is unclear if this was due to DHA or various confounding factors. The tolerable upper intake level of DHA during pregnancy or lactation has not been established; most experts recommend DHA 200-300 mg daily. While it is typically advised that this need is met by consuming 8-12 ounces of seafood weekly during pregnancy and 4-8 ounces weekly during lactation, those with nutrient deficiency or those following a vegan diet may meet this need with supplementation (95740,95741).
Below is general information about the interactions of the known ingredients contained in the product Omega-3 Vegetarian DHA 200 mg. Some ingredients may not be listed. This information does NOT represent a recommendation for or a test of this specific product as a whole.
Theoretically, DHA may increase the risk of bleeding if used with anticoagulant or antiplatelet drugs.
Although some clinical evidence suggests that DHA might reduce collagen-stimulated platelet aggregation and thromboxane release, most clinical evidence suggests that DHA alone does not affect blood clotting (11112,11113,48020). However, theoretically, when given in combination with EPA as fish oil, concomitant use with anticoagulant or antiplatelet drugs (including aspirin) might increase risk of bleeding.
|
Theoretically, taking DHA with antidiabetes drugs might reduce the effects of these medications.
In people with type 2 diabetes, including those taking oral hypoglycemic medications, DHA seems to increase fasting blood glucose levels (10321).
|
Theoretically, taking DHA with antihypertensive drugs might increase the risk of hypotension.
|
Below is general information about the adverse effects of the known ingredients contained in the product Omega-3 Vegetarian DHA 200 mg. Some ingredients may not be listed. This information does NOT represent a recommendation for or a test of this specific product as a whole.
General
...Orally, DHA is generally well-tolerated when used in doses up to 3 grams daily.
Intravenously, DHA seems to be well tolerated.
Most Common Adverse Effects:
Orally: Belching, fishy aftertaste, loose stools, and nausea.
Serious Adverse Effects (Rare):
Orally: Some case reports raise concerns about increased risk of bleeding with high doses of fish oil containing DHA.
Cardiovascular ...Orally, DHA might increase low-density lipoprotein (LDL) cholesterol levels. However, this appears to be primarily due to increases in the large buoyant type of LDL particles. The small, dense type of LDL particles are reduced (6143,48013,48078,48083,48174,48338).
Dermatologic ...Orally, DHA has been associated with one report of rash and one report of warmth on hands in one clinical study (48217). In another clinical study, two patients taking DHA 400 mg daily reported acne (11333). In another clinical study, one parent of a pediatric patient treated with DHA 600 mg daily reported increased hair loss beginning 6 weeks after completion of supplementation (90699). It is unclear if this adverse effect is specifically related to DHA intake.
Gastrointestinal
...Orally, DHA may cause gastrointestinal upset, fishy aftertaste, belching, flatulence, heartburn, loose stools, anorexia, and dry mouth (10869,11333,48217,109218).
There is also some evidence that increased serum levels of DHA might be associated with an increased risk for atrophic gastritis associated with Helicobacter pylori infection, but further research is needed to clarify this finding (8709).
For fish oils containing EPA and DHA, side effects can include fishy taste, belching, nausea, and loose stools (1009,1313,8699,10007). Three people with pre-existing familial adenomatous polyposis were diagnosed with malignant lesions during the course of long-term fish oil use (999).
Genitourinary ...Orally, one patient in one clinical study who was taking DHA 1, 2, or 4 grams daily (specific dose unclear) reported decreased libido (48217).
Hematologic ...Orally, DHA might cause nose bleeds, but this is uncommon. Onset of severe nose bleeds has been reported in one clinical study in one child who took DHA 600 mg daily (98542). Although most clinical research shows that DHA does not affect blood clotting when taken alone (11112,11113,48020), there is some concern that taking high doses of oils providing DHA along with eicosapentaenoic acid (EPA) might decrease blood coagulation and increase the risk of bleeding (1313). The US Food and Drug Administration (FDA) recommends that consumers limit intake of EPA plus DHA to 3 grams daily, with no more than 2 grams daily from a dietary supplement (95739).
Neurologic/CNS ...Orally, DHA may cause dizziness, headache, insomnia, fatigue, and anxiety (10869,11333,48217). In one clinical study, one parent of a pediatric patient treated with DHA 600 mg daily reported increased disruptive behavior in the child (90699).
Ocular/Otic ...Orally, DHA may cause watery eyes but results are inconsistent. In one clinical study, five of 167 infants fed formula containing 0.32% or 0.64% DHA experienced watery eyes. However, none of the infants fed formula containing 0.96% DHA experienced watery eyes (90670). In one clinical study, one patient taking DHA 400 mg daily experienced an ear infection. It is unclear if this event was related to DHA supplementation.
Oncologic ...Orally, DHA may increase the risk of prostate cancer, but additional research is needed to clarify this finding. A meta-analysis of data from observational studies found that higher dietary intake of DHA is associated with a non-linear increased risk of prostate cancer (90677). It is unclear if supplemental DHA intake is associated with increased risk of prostate cancer.
Pulmonary/Respiratory ...Orally, worsened asthma symptoms were reported by one parent of one patient with asthma taking DHA 600 mg daily (90699).
