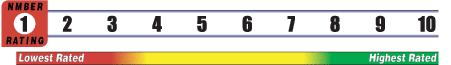
| Ingredients | Amount Per Serving |
|---|---|
|
(Pueraria lobata )
(Root)
|
150 mg |
|
(Lobelia inflata, Lobelia inflata, Lobelia inflata)
|
90 mg |
|
(Paeonia lactiflora Concentrate)
(4:1 concentrate equivalent to approximately 3,000 mg of fresh herb)
|
90 mg |
|
(Cinnamomum verum Concentrate)
(4:1 concentrate equivalent to approximately 3,000 mg of fresh herb)
|
90 mg |
|
(root)
(Glycyrrhiza glabra Root Concentrate)
(4:1 concentrate equivalent to approximately 3,000 mg of fresh herb)
|
65 mg |
|
(Schisandra chinensis Concentrate)
(4:1 concentrate equivalent to approximately 3,000 mg of fresh herb )
|
60 mg |
|
(Zingiber officinale )
(root)
|
45 mg |
|
Xanthium
(Xanthium sibiricum)
|
40 mg |
|
(flower)
(Magnolia bioundii Concentrate)
(4:1 concentrate equivalent to approximately 3,000 mg of fresh herb)
|
30 mg |
Capsule, Stearate
Below is general information about the effectiveness of the known ingredients contained in the product AllerEze. Some ingredients may not be listed. This information does NOT represent a recommendation for or a test of this specific product as a whole.
INSUFFICIENT RELIABLE EVIDENCE to RATE
INSUFFICIENT RELIABLE EVIDENCE to RATE
INSUFFICIENT RELIABLE EVIDENCE to RATE
INSUFFICIENT RELIABLE EVIDENCE to RATE
INSUFFICIENT RELIABLE EVIDENCE to RATE
INSUFFICIENT RELIABLE EVIDENCE to RATE
INSUFFICIENT RELIABLE EVIDENCE to RATE
INSUFFICIENT RELIABLE EVIDENCE to RATE
Below is general information about the safety of the known ingredients contained in the product AllerEze. Some ingredients may not be listed. This information does NOT represent a recommendation for or a test of this specific product as a whole.
LIKELY SAFE ...when consumed in amounts commonly found in foods. Cassia cinnamon has Generally Recognized As Safe (GRAS) status in the US for use as a spice or flavoring agent (4912) ...when used orally and appropriately, short-term. Cassia cinnamon up to 2 grams daily has been used safely for up to 3 months (17011,21914). Cassia cinnamon 3-6 grams daily has been used safely for up to 6 weeks (11347,14344). Cassia cinnamon extract corresponding to 3 grams daily of cassia cinnamon powder has also been used safely for up to 4 months (21916).
POSSIBLY SAFE ...when used topically, short-term. Cassia cinnamon oil 5% cream applied topically to the legs has been used safely in one clinical trial (59580).
POSSIBLY UNSAFE ...when used orally in high doses, long-term. Some cassia cinnamon products contain high levels of coumarin. Coumarin can cause hepatotoxicity in animal models (15299,21920). In humans, very high doses of coumarin from 50-7000 mg daily can result in hepatotoxicity that resolves when coumarin use is discontinued (15302). In most cases, ingestion of cassia cinnamon will not provide a high enough amount of coumarin to cause significant toxicity; however, in especially sensitive people, such as those with liver disease, prolonged ingestion of large amounts of cassia cinnamon might exacerbate the condition.
CHILDREN: POSSIBLY SAFE
when used orally and appropriately, short-term.
Cassia cinnamon 1 gram daily has been used safely in adolescents 13-18 years of age for up to 3 months (89648).
PREGNANCY AND LACTATION: LIKELY SAFE
when consumed in amounts commonly found in foods (4912).
There is insufficient reliable information available about the safety of cassia cinnamon when used in medicinal amounts during pregnancy and breast-feeding. Stay on the safe side and stick to food amounts.
LIKELY SAFE ...when consumed in amounts commonly found in foods. Ceylon cinnamon has Generally Recognized As Safe (GRAS) status in the US for use as a spice or flavoring agent (4912).
POSSIBLY SAFE ...when used orally and appropriately in medicinal amounts. Ceylon cinnamon 0.5-3 grams daily has been safely used in studies lasting up to 6 months (4,12,97248,97250,99874). ...when used as a mouth rinse for up to 15 days (92071). There is insufficient reliable information available about the safety of Ceylon cinnamon when used orally in greater amounts or for longer periods. Ceylon cinnamon contains trace amounts of coumarin (108260). In very high doses, coumarin can cause hepatotoxicity (15302). However, since the amount of coumarin in Ceylon cinnamon is negligible, it is unlikely to cause toxic effects (89652,92072,92073).
PREGNANCY: LIKELY SAFE
when consumed in amounts commonly found in foods (4912).
PREGNANCY: LIKELY UNSAFE
when used orally in amounts greater than those found in foods.
Fetal abnormalities have been reported in animals (4,12).
LACTATION: LIKELY SAFE
when consumed in amounts commonly found in foods (4912).
There is insufficient reliable information available about the safety of Ceylon cinnamon in amounts greater than those found in foods.
LIKELY SAFE ...when used orally and appropriately. Ginger has been safely used in multiple clinical trials (721,722,723,5343,7048,7084,7085,7400,7623,11346)(12472,13080,13237,13244,17369,17928,17929,89889,89890,89894)(89895,89898,89899,90102,96252,96253,96259,96260,96669) (101760,101761,101762,103359,107903).
POSSIBLY SAFE ...when used topically and appropriately, short-term (89893,89897).
CHILDREN: LIKELY SAFE
when consumed in the amounts typically found in foods.
CHILDREN: POSSIBLY SAFE
when used orally and appropriately, short-term.
Ginger powder has been used with apparent safety at a dose of up to 750 mg daily for 4 days in girls aged 14-18 years (96255).
PREGNANCY: LIKELY SAFE
when consumed in the amounts typically found in foods.
Ginger is considered a first-line nonpharmacological treatment option for nausea in pregnancy by the American College of Obstetrics and Gynecology (ACOG) (111601). However, it should not be used long-term or without medical supervision and close monitoring.
PREGNANCY: POSSIBLY SAFE
when used for medicinal purposes.
Despite some early reports of adverse effects (721,7083) and one observational study suggesting that taking dried ginger and other herbal supplements during the first 20 weeks of pregnancy marginally increased the chance of stillbirth (96254), most research shows that ginger is unlikely to cause harm to the baby. The risk for major malformations in infants of parents who took ginger when pregnant does not appear to be higher than the baseline rate of 1% to 3% (721,1922,5343,11346,13071,13080,96254). Also, other research suggests that ginger intake during various trimesters does not significantly affect the risk of spontaneous abortion, congenital malformations, stillbirth, perinatal death, preterm birth, low birth weight, or low Apgar scores (18211,90103). Ginger use has been associated with an increase in non-severe vaginal bleeding, including spotting, after week 17 of pregnancy (18211).
LACTATION: LIKELY SAFE
when consumed in the amounts typically found in foods.
There is insufficient reliable information available about the safety of ginger when used for medicinal purposes; avoid amounts greater than those found in foods.
POSSIBLY SAFE ...when used orally and appropriately. Kudzu appears to be safe for up to 4 months (10386,11386,92257). ...when used intravaginally and appropriately. Kudzu 5% to 6% gel has been used with apparent safety for up to 12 weeks (96740,105521,110702).
PREGNANCY AND LACTATION:
Insufficient reliable information available; avoid using.
LIKELY SAFE ...when used orally in amounts commonly found in foods. Licorice has Generally Recognized as Safe (GRAS) status in the US (4912).
POSSIBLY SAFE ...when licorice products that do not contain glycyrrhizin (deglycyrrhizinated licorice) are used orally and appropriately for medicinal purposes. Licorice flavonoid oil 300 mg daily for 16 weeks, and deglycyrrhizinated licorice products in doses of up to 4.5 grams daily for up to 16 weeks, have been used with apparent safety (6196,11312,11313,17727,100984,102960). ...when licorice products containing glycyrrhizin are used orally in low doses, short-term. Licorice extract 272 mg, containing glycyrrhizin 24.3 mg, has been used daily with apparent safety for 6 months (102961). A licorice extract 1000 mg, containing monoammonium glycyrrhizinate 240 mg, has been used daily with apparent safety for 12 weeks (110320). In addition, a syrup providing licorice extract 750 mg has been used twice daily with apparent safety for 5 days (104558). ...when applied topically. A gel containing 2% licorice root extract has been applied to the skin with apparent safety for up to 2 weeks. (59732). A mouth rinse containing 5% licorice extract has been used with apparent safety four times daily for up to one week (104564).
POSSIBLY UNSAFE ...when licorice products containing glycyrrhizin are used orally in large amounts for several weeks, or in smaller amounts for longer periods of time. The European Scientific Committee on Food recommends that a safe average daily intake of glycyrrhizin should not exceed 10 mg (108577). In otherwise healthy people, consuming glycyrrhizin daily for several weeks or longer can cause severe adverse effects including pseudohyperaldosteronism, hypertensive crisis, hypokalemia, cardiac arrhythmias, and cardiac arrest. Doses of 20 grams or more of licorice products, containing at least 400 mg glycyrrhizin, are more likely to cause these effects; however, smaller amounts have also caused hypokalemia and associated symptoms when taken for months to years (781,3252,15590,15592,15594,15596,15597,15599,15600,16058)(59731,59740,59752,59785,59786,59787,59792,59795,59805,59811)(59816,59818,59820,59822,59826,59828,59849,59850,59851,59867)(59882,59885,59888,59889,59895,59900,59906,97213,110305). In patients with hypertension, cardiovascular or kidney conditions, or a high salt intake, as little as 5 grams of licorice product or 100 mg glycyrrhizin daily can cause severe adverse effects (15589,15593,15598,15600,59726).
PREGNANCY: UNSAFE
when used orally.
Licorice has abortifacient, estrogenic, and steroid effects. It can also cause uterine stimulation. Heavy consumption of licorice, equivalent to 500 mg of glycyrrhizin per week (about 250 grams of licorice per week), during pregnancy seems to increase the risk of delivery before gestational age of 38 weeks (7619,10618). Furthermore, high intake of glycyrrhizin, at least 500 mg per week, during pregnancy is associated with increased salivary cortisol levels in the child by the age of 8 years. This suggests that high intake of licorice during pregnancy may increase hypothalamic-pituitary-adrenocortical axis activity in the child (26434); avoid using.
LACTATION:
Insufficient reliable information available; avoid using.
LIKELY UNSAFE ...when used orally (3,11). Lobelia leaf can be toxic in doses of 600-1000 mg; 4000 mg of the leaf may be fatal (18). There is insufficient reliable information available about the safety of lobelia when used topically.
PREGNANCY AND LACTATION: LIKELY UNSAFE
when used orally due to its emetic effects (4,12).
There is insufficient reliable information available about the safety of lobelia when used topically during pregnancy and lactation.
POSSIBLY SAFE ...when used orally and appropriately, short-term. A specific product containing magnolia extract and phellodendron extract (Relora, Next Pharmaceuticals, Inc.) has been used with apparent safety in clinical trials at a dose of 250 mg two to three times daily for up to 6 weeks (14349,34246,94904). ...when used topically in a toothpaste for up to 6 months (92464).
PREGNANCY: UNSAFE
when the magnolia flower bud is used orally due to reports of uterine stimulant activity (11953).
There is insufficient reliable information available about the safety of using magnolia bark during pregnancy; avoid using.
LACTATION:
Insufficient reliable information available; avoid using.
POSSIBLY SAFE ...when used orally and appropriately, short term. Total glucosides of peony has been used with apparent safety in doses of up to 1800 mg daily for up to 12 months (92786,97949,97950,98466,100992,110432,112861,112862). Peony root extract has been used with apparent safety at a dose of 2250 mg daily for up to 3 months (97216). There is insufficient reliable information available about the safety of peony when used orally, topically, or rectally, long-term.
CHILDREN: POSSIBLY SAFE
when used orally and appropriately, short-term.
Total glucosides of peony has been used with apparent safety in children 1.5-4 years of age at doses up to 180 mg/kg daily or 1.2 grams daily for up to 12 months (92785). Peony root extract 40 mg/kg daily has also been used with apparent safety in children 1-14 years of age for 4 weeks (106851).
PREGNANCY: POSSIBLY UNSAFE
when used orally.
Preliminary research suggests that peony can cause uterine contractions (13400). However, other preliminary research suggests a combination of peony and angelica with or without motherwort, banksias rose, and ligustica, might be safe (11015,48433). Until more is known, avoid use.
LACTATION:
Insufficient reliable information available; avoid using.
POSSIBLY SAFE ...when used orally and appropriately. Schisandra extract up to 1 gram daily has been used for up to 12 weeks with apparent safety (12,96632,105562,105563,112887).
PREGNANCY: POSSIBLY UNSAFE
when used orally.
Some evidence suggests schisandra fruit is a uterine stimulant (11).
LACTATION:
Insufficient reliable information available; avoid using.
Below is general information about the interactions of the known ingredients contained in the product AllerEze. Some ingredients may not be listed. This information does NOT represent a recommendation for or a test of this specific product as a whole.
Theoretically, cassia cinnamon may have additive effects with antidiabetes drugs.
|
Theoretically, large doses of cassia cinnamon might cause additive effects when used with hepatotoxic drugs.
There is some concern that ingesting large amounts of cassia cinnamon for an extended duration might cause hepatotoxicity in some people. Cassia cinnamon contains coumarin, which can cause hepatotoxicity in animal models (15299,21920). In humans, very high doses of coumarin from 50-7000 mg/day can result in hepatotoxicity that resolves when coumarin use is discontinued (15302,97249). Lower amounts might also cause liver problems in sensitive people, such as those with liver disease or those taking potentially hepatotoxic agents.
|
Theoretically, Ceylon cinnamon may have additive effects with antidiabetes drugs.
|
Theoretically, Ceylon cinnamon might have additive effects with antihypertensive drugs and increase the risk of hypotension.
|
Ginger may have antiplatelet effects and may increase the risk of bleeding if used with anticoagulant or antiplatelet drugs. However, research is conflicting.
Laboratory research suggests that ginger inhibits thromboxane synthetase and decreases platelet aggregation (7622,12634,20321,20322,20323,96257). However, this has not been demonstrated unequivocally in humans, with mixed results from clinical trials (96257). Theoretically, excessive amounts of ginger might increase the risk of bleeding when used with anticoagulant/antiplatelet drugs.
|
Theoretically, taking ginger with antidiabetes drugs might increase the risk of hypoglycemia.
|
Theoretically, taking ginger with calcium channel blockers might increase the risk of hypotension.
Some animal and in vitro research suggests that ginger has hypotensive and calcium channel-blocking effects (12633). Another animal study shows that concomitant administration of ginger and the calcium channel blocker amlodipine leads to greater reductions in blood pressure when compared with amlodipine alone (107901).
|
Theoretically, when taken prior to cyclosporine, ginger might decrease cyclosporine levels.
In an animal model, ginger juice taken 2 hours prior to cyclosporine administration reduced the maximum concentration and area under the curve of cyclosporine by 51% and 40%, respectively. This effect was not observed when ginger juice and cyclosporine were administered at the same time (20401).
|
Theoretically, ginger might increase the levels of CYP1A2 substrates.
In vitro research shows that ginger inhibits CYP1A2 activity (111544). However, this interaction has not been reported in humans.
|
Theoretically, ginger might increase the levels of CYP2B6 substrates.
In vitro research shows that ginger inhibits CYP2B6 activity (111544). However, this interaction has not been reported in humans.
|
Theoretically, ginger might increase the levels of CYP2C9 substrates.
In vitro research shows that ginger inhibits CYP2C9 activity (111544). However, this interaction has not been reported in humans.
|
Ginger might increase or decrease the levels of CYP3A4 substrates.
In vitro research and some case reports suggest that ginger inhibits CYP3A4 activity (111544,111644). Three case reports from the World Health Organization (WHO) adverse drug reaction database describe increased toxicity in patients taking ginger and cancer medications that are CYP3A4 substrates (imatinib, dabrafenib, and crizotinib). However, the causality of this interaction is unclear due to the presence of multiple interacting drugs and routes of administration (111644).
Conversely, other in vitro research suggests that ginger induces CYP3A4 activity, leading to reduced levels of CYP3A4 substrates (111404). However, this interaction has not been reported in humans. |
Theoretically, ginger might increase levels of losartan and the risk of hypotension.
In animal research, ginger increased the levels and hypotensive effects of a single dose of losartan (102459). It is not clear if ginger alters the concentration or effects of losartan when taken continuously. Additionally, this interaction has not been shown in humans.
|
Theoretically, ginger might increase levels of metronidazole.
In an animal model, ginger increased the absorption and plasma half-life of metronidazole. In addition, the elimination rate and clearance of metronidazole was significantly reduced (20350).
|
Ginger may have antiplatelet effects and increase the risk of bleeding if used with nifedipine.
Clinical research shows that combined treatment with ginger 1 gram plus nifedipine 10 mg significantly inhibits platelet aggregation when compared to nifedipine or ginger alone (20324).
|
Ginger might increase the absorption and blood levels of P-glycoprotein (P-gp) substrates.
In vitro research and case reports suggest that ginger inhibits drug efflux by P-gp, potentially increasing absorption and serum levels of P-gp substrates (111544,111644). Two case reports from the World Health Organization (WHO) adverse drug reaction database describe increased toxicity in patients taking ginger and cancer medications that are P-gp substrates (trametinib, crizotinib). However, the causality of this interaction is unclear due to the presence of multiple interacting drugs and routes of administration (111644).
|
Ginger might increase the risk of bleeding with phenprocoumon.
Phenprocoumon, a warfarin-related anticoagulant, might increase the international normalized ratio (INR) when taken with ginger. There is one case report of a 76-year-old woman with a stable INR on phenprocoumon that increased to greater than 10 when she began consuming dried ginger and ginger tea (12880).
|
Ginger might increase the risk of bleeding with warfarin.
Laboratory research suggests that ginger might inhibit thromboxane synthetase and decrease platelet aggregation (7622,12634,20321,20322,20323). In one case report, ginger increased the INR when taken with phenprocoumon, which has similar pharmacological effects as warfarin (12880). In another case report, ginger increased the INR when taken with a combination of warfarin, hydrochlorothiazide, and acetaminophen (20349). A longitudinal analysis suggests that taking ginger increases the risk of bleeding in patients taking warfarin for at least 4 months (20348). However, research in healthy people suggests that ginger has no effect on INR, or the pharmacokinetics or pharmacodynamics of warfarin (12881,15176). Until more is known, monitor INRs closely in patients taking large amounts of ginger.
|
Theoretically, kudzu may increase the risk of bleeding if used with antiplatelet or anticoagulant drugs.
|
Theoretically, taking kudzu with antidiabetes drugs might increase the risk of hypoglycemia.
|
Theoretically, taking kudzu with caffeine might increase levels of caffeine.
In healthy males injected with the kudzu constituent puerarin, caffeine clearance and metabolism is inhibited (23583). This effect has been attributed to inhibition of cytochrome P450 1A2 (CYP1A2) enzyme, which is involved in caffeine metabolism. It is unclear if taking kudzu orally would have this same effect.
|
Theoretically, kudzu might alter the effects of estrogen therapy.
|
Theoretically, concomitant use might have additive hepatotoxic effects.
|
Theoretically, taking kudzu with methotrexate might increase the risk of methotrexate toxicity.
Preclinical research suggests that kudzu extract greatly reduces the elimination and increases the toxicity of methotrexate. Kudzu might inhibit organic anion transporters (OATs) that are responsible for hepatobiliary and renal excretion of anions, similar to the interaction between methotrexate and non-steroidal anti-inflammatory drugs (NSAIDs) (13296).
|
Theoretically, kudzu might interfere with tamoxifen activity.
|
Theoretically, licorice might reduce the effects of antihypertensive drugs.
|
Theoretically, licorice might reduce the effects of cisplatin.
In animal research, licorice diminished the therapeutic efficacy of cisplatin (59763).
|
Theoretically, concomitant use of licorice and corticosteroids might increase the side effects of corticosteroids.
Case reports suggest that concomitant use of licorice and oral corticosteroids, such as hydrocortisone, can potentiate the duration of activity and increase blood levels of corticosteroids (3252,12672,20040,20042,48429,59756). Additionally, in one case report, a patient with neurogenic orthostatic hypertension stabilized on fludrocortisone 0.1 mg twice daily developed pseudohyperaldosteronism after recent consumption of large amounts of black licorice (108568).
|
Theoretically, licorice might decrease the levels and clinical effects of CYP1A2 substrates.
In vitro research shows that licorice induces CYP1A2 enzymes (111404).
|
Theoretically, licorice might increase levels of drugs metabolized by CYP2B6.
In vitro research shows that licorice extract and glabridin, a licorice constituent, inhibit CYP2B6 isoenzymes (10300,94822). Licorice extract from the species G. uralensis seems to inhibit CYP2B6 isoenzymes to a greater degree than G. glabra extract in vitro (94822). Theoretically, these species of licorice might increase levels of drugs metabolized by CYP2B6; however, these interactions have not yet been reported in humans.
|
Theoretically, licorice might increase levels of drugs metabolized by CYP2C19.
In vitro, licorice extracts from the species G. glabra and G. uralensis inhibit CYP2C19 isoenzymes in vitro (94822). Theoretically, these species of licorice might increase levels of drugs metabolized by CYP2C19; however, this interaction has not yet been reported in humans.
|
Theoretically, licorice might increase levels of drugs metabolized by CYP2C8.
In vitro, licorice extract from the species G. glabra and G. uralensis inhibits CYP2C8 isoenzymes (94822). Theoretically, these species of licorice might increase levels of drugs metabolized by CYP2C8; however, this interaction has not yet been reported in humans.
|
Theoretically, licorice might increase or decrease levels of drugs metabolized by CYP2C9.
There is conflicting evidence about the effect of licorice on CYP2C9 enzyme activity. In vitro research shows that extracts from the licorice species G. glabra and G. uralensis moderately inhibit CYP2C9 isoenzymes (10300,94822). However, evidence from an animal model shows that licorice extract from the species G. uralensis can induce hepatic CYP2C9 activity (14441). Until more is known, licorice should be used cautiously in people taking CYP2C9 substrates.
|
Theoretically, licorice might increase or decrease levels of drugs metabolized by CYP3A4.
Pharmacokinetic research shows that the licorice constituent glycyrrhizin, taken in a dosage of 150 mg orally twice daily for 14 days, modestly decreases the area under the concentration-time curve of midazolam by about 20%. Midazolam is a substrate of CYP3A4, suggesting that glycyrrhizin modestly induces CYP3A4 activity (59808). Animal research also shows that licorice extract from the species G. uralensis induces CYP3A4 activity (14441). However, licorice extract from G. glabra species appear to inhibit CYP3A4-induced metabolism of testosterone in vitro. It is thought that the G. glabra inhibits CYP3A4 due to its constituent glabridin, which is a moderate CYP3A4 inhibitor in vitro and not present in other licorice species (10300,94822). Until more is known, licorice should be used cautiously in people taking CYP3A4 substrates.
|
Theoretically, concomitant use of licorice with digoxin might increase the risk of cardiac toxicity.
Overuse or misuse of licorice with cardiac glycoside therapy might increase the risk of cardiac toxicity due to potassium loss (10393).
|
Theoretically, concomitant use of licorice with diuretic drugs might increase the risk of hypokalemia.
Overuse of licorice might compound diuretic-induced potassium loss (10393,20045,20046,59812). In one case report, a 72-year-old male with a past medical history of hypertension, type 2 diabetes, hyperlipidemia, arrhythmia, stroke, and hepatic dysfunction was hospitalized with severe hypokalemia and uncontrolled hypertension due to pseudohyperaldosteronism. This was thought to be provoked by concomitant daily consumption of a product containing 225 mg of glycyrrhizin, a constituent of licorice, and hydrochlorothiazide 12.5 mg for 1 month (108577).
|
Theoretically, licorice might increase or decrease the effects of estrogen therapy.
|
Theoretically, loop diuretics might increase the mineralocorticoid effects of licorice.
Theoretically, loop diuretics might enhance the mineralocorticoid effects of licorice by inhibiting the enzyme that converts cortisol to cortisone; however, bumetanide (Bumex) does not appear to have this effect (3255).
|
Theoretically, licorice might increase levels of methotrexate.
Animal research suggests that intravenous administration of glycyrrhizin, a licorice constituent, and high-dose methotrexate may delay methotrexate excretion and increase systemic exposure, leading to transient elevations in liver enzymes and total bilirubin (108570). This interaction has not yet been reported in humans.
|
Theoretically, licorice might decrease levels of midazolam.
In humans, the licorice constituent glycyrrhizin appears to moderately induce the metabolism of midazolam (59808). This is likely due to induction of cytochrome P450 3A4 by licorice. Until more is known, licorice should be used cautiously in people taking midazolam.
|
Theoretically, licorice might decrease the absorption of P-glycoprotein substrates.
In vitro research shows that licorice can increase P-glycoprotein activity (104561).
|
Theoretically, licorice might decrease plasma levels and clinical effects of paclitaxel.
Multiple doses of licorice taken concomitantly with paclitaxel might reduce the effectiveness of paclitaxel. Animal research shows that licorice 3 grams/kg given orally for 14 days before intravenous administration of paclitaxel decreases the exposure to paclitaxel and increases its clearance. Theoretically, this occurs because licorice induces cytochrome P450 3A4 enzymes, which metabolize paclitaxel. Notably, a single dose of licorice did not affect exposure or clearance of paclitaxel (102959).
|
Theoretically, licorice might decrease plasma levels and clinical effects of warfarin.
Licorice seems to increase metabolism and decrease levels of warfarin in animal models. This is likely due to induction of cytochrome P450 2C9 (CYP2C9) metabolism by licorice (14441). Advise patients taking warfarin to avoid taking licorice.
|
Lobelia is thought to have diuretic properties. Theoretically, due to these potential diuretic effects, lobelia might reduce excretion and increase levels of lithium. The dose of lithium might need to be decreased.
|
Theoretically, magnolia might have additive effects and increase the risk of bleeding when used with anticoagulant or antiplatelet drugs.
In vitro research shows that the chemicals magnolol and honokiol, isolated from magnolia bark, inhibit platelet aggregation that is experimentally induced by collagen and arachidonic acid. However, they do not inhibit platelet aggregation that is induced by adenosine diphosphate, platelet-activating factor, or thrombin (18273). This interaction has not been reported in humans.
|
Theoretically, concomitant use of large doses of magnolia bark and CNS depressants might have additive effects.
|
Theoretically, combining peony with anticoagulant or antiplatelet drugs might increase the risk of bleeding.
In vitro research suggests that peony might have antiplatelet, anticoagulant, and antithrombotic effects (92787).
|
Theoretically, peony might increase the levels and clinical effects of clozapine.
In vitro research shows that peony suppresses the metabolism of clozapine via weak-to-moderate inhibitory effects on cytochromes P450 (CYP) 1A2 and CYP3A4 (92790). This effect has not been reported in humans.
|
Theoretically, peony might interfere with contraceptive drugs due to competition for estrogen receptors.
In vitro and animal research shows that peony extract has estrogenic activity (100990). Concomitant use might also increase the risk for estrogen-related adverse effects.
|
Theoretically, use of peony may increase the levels and clinical effects of drugs metabolized by CYP1A2.
In vitro research shows that peony suppresses the metabolism of clozapine via weak-to-moderate inhibitory effects on CYP1A2 and CYP3A4 (92790). This effect has not been reported in humans.
|
Theoretically, use of peony may increase the levels and clinical effects of drugs metabolized by CYP3A4.
In vitro research shows that peony suppresses the metabolism of clozapine via weak-to-moderate inhibitory effects on CYP1A2 and CYP3A4 (92790). This effect has not been reported in humans.
|
Theoretically, concomitant use of large amounts of peony might interfere with hormone replacement therapy and/or increase the risk for estrogen-related adverse effects.
In vitro and animal research shows that peony extract has estrogenic activity (100990). Theoretically, peony might compete for estrogen receptors and/or cause additive estrogenic effects.
|
Theoretically, peony might reduce the levels and clinical effects of phenytoin.
Animal research shows that taking peony root reduces levels of phenytoin (8657). Some researchers suggest that peony root might affect cytochrome P450 (CYP) 2C9, which metabolizes phenytoin. However, preliminary research in humans shows that peony root does not alter levels of losartan (Cozaar), which is also metabolized by CYP2C9 (11480).
|
Theoretically, schisandra might increase the levels and clinical effects of cyclophosphamide.
In vitro research shows that schisandra increases the concentration of cyclophosphamide, likely through inhibition of cytochrome P450 3A4. After multiple doses of the schisandra constituents schisandrin A and schisantherin A, the maximum concentration of cyclophosphamide was increased by 7% and 75%, respectively, while the overall exposure to cyclophosphamide was increased by 29% and 301%, respectively (109636).
|
Schisandra can increase the levels and clinical effects of cyclosporine.
A small observational study in children with aplastic anemia found that taking schisandra with cyclosporine increased cyclosporine trough levels by 93% without increasing the risk of adverse events. However, the dose of cyclosporine was reduced in 9% of children to maintain appropriate cyclosporine blood concentrations (109637).
|
Theoretically, schisandra might increase the levels and clinical effects of CYP2C19 substrates.
In vitro research shows that schisandra inhibits CYP2C19, and animal research shows that schisandra increases the concentration of voriconazole, a CYP2C19 substrate (105566). Theoretically, schisandra may also inhibit the metabolism of other CYP2C19 substrates. This effect has not been reported in humans.
|
Theoretically, schisandra might decrease the levels and clinical effects of CYP2C9 substrates.
In vitro and animal research suggests that schisandra induces CYP2C9 enzymes (14441). This effect has not been reported in humans.
|
Schisandra can increase the levels and clinical effects of drugs metabolized by CYP3A4.
Most clinical and laboratory research shows that schisandra, administered either as a single dose or up to twice daily for 14 days, inhibits CYP3A4 and increases the concentration of CYP3A4 substrates such as cyclophosphamide, midazolam, tacrolimus, and talinolol (13220,17414,23717,91386,91388,91387,96631,105564,109636,109638,109639,109640,109641). Although one in vitro and animal study shows that schisandra may induce CYP3A4 metabolism (14441), this effect appears to be overpowered by schisandra's CYP3A4 inhibitory activity and has not been reported in humans.
|
Schisandra can increase the levels and clinical effects of midazolam.
A small pharmacokinetic study in healthy adults shows that taking schisandra extract (Hezheng Pharmaceutical Co.) containing deoxyschizandrin 33.75 mg twice daily for 8 days and a single dose of midazolam 15 mg on day 8 increases the overall exposure to midazolam by about 119%, increases the peak plasma level of midazolam by 86%, and decreases midazolam clearance by about 52%. This effect has been attributed to inhibition of CYP3A4 by schisandra (91388).
|
Schisandra might increase the levels and clinical effects of P-glycoprotein substrates.
In vitro research shows that schisandra extracts and constituents such as schisandrin B inhibit P-glycoprotein mediated efflux in intestinal cells and in P-glycoprotein over-expressing cell lines (17414,105643,105644). Additionally, a small clinical study shows that schisandra increases the peak concentration and overall exposure to talinolol, a P-glycoprotein probe substrate (91386). Theoretically, schisandra might inhibit the efflux of other P-glycoprotein substrates.
|
Schisandra can increase the levels and clinical effects of sirolimus.
A small pharmacokinetic study in healthy volunteers shows that taking 3 capsules of schisandra (Hezheng Pharmaceutical Company) containing a total of 33.75 mg deoxyschizandrin twice daily for 13 days and then taking a single dose of sirolimus 2 mg increases the overall exposure and peak level of sirolimus by two-fold. This effect is thought to be due to inhibition of cytochrome P450 3A4 by schisandra, as well as possible inhibition of the P-glycoprotein drug transporter (105643).
|
Schisandra can increase the levels and clinical effects of tacrolimus.
Clinical research in healthy children and adults, transplant patients, and patients with nephrotic syndrome and various rheumatic immunologic disorders shows that taking schisandra with tacrolimus increases tacrolimus peak levels by 183% to 268%, prolongs or delays time to peak tacrolimus concentrations, increases overall exposure to tacrolimus by 126% to 343%, and decreases tacrolimus clearance by 19% to 73% (17414,91387,15570,96631,105623,109638,109639,109640,109641,112889)(112890,112972,112973,112974). This effect is thought to be due to inhibition of P-glycoprotein drug transporter and CYP3A4 and CYP3A5 by schisandra (17414,96631,105623,105643,105644,112974). Some clinical and observational studies suggest that schisandra increases tacrolimus levels similarly in both expressors and non-expressors of CYP3A5, while other studies suggest it does so to a greater degree in CYP3A5 expressors than non-expressors (105623,109638,109639,109640,112889,112890,112973,112974). Animal research suggests that the greatest increase in tacrolimus levels occurs when schisandra is taken either concomitantly or up to 2 hours before tacrolimus (105564), and clinical and observational research in humans suggests that schisandra may increase whole blood levels of tacrolimus and decrease clearance of tacrolimus in a dose-dependent manner (109639,109640,112972).
|
Schisandra can increase the levels and clinical effects of talinolol.
A small pharmacokinetic study in healthy volunteers shows that taking schisandra extract 300 mg twice daily for 14 days with a single dose of talinolol 100 mg on day 14 increases the peak talinolol level by 51% and the overall exposure to talinolol by 47%. This effect is thought to be due to the possible inhibition of cytochrome P450 3A4 and P-glycoprotein by schisandra (91386).
tly.
|
Theoretically, schisandra might increase the levels and clinical effects of voriconazole.
Animal research shows that oral schisandra given daily for 1 or 14 days increases levels of intravenously administered voriconazole, a cytochrome P450 (CYP) 2C19 substrate. This effect is thought to be due to inhibition of CYP2C19 by schisandra (105566). However, this interaction has not been reported in humans.
|
Theoretically, schisandra might decrease the levels and clinical effects of warfarin.
Animal research suggests that oral schisandra extract, given daily for 6 days, reduces levels of intravenously administered warfarin. This effect might be due to the induction of cytochrome P450 (CYP) 2C9 metabolism by schisandra (14441). However, this interaction has not been reported in humans.
|
Below is general information about the adverse effects of the known ingredients contained in the product AllerEze. Some ingredients may not be listed. This information does NOT represent a recommendation for or a test of this specific product as a whole.
General
...Orally, cassia cinnamon appears to be well-tolerated.
Significant side effects have not been reported in most patients.
Most Common Adverse Effects:
Topically: Burning mouth, stomatitis.
Dermatologic
...In one clinical trial, a rash was reported in one patient taking cassia cinnamon 1 gram daily for 90 days (17011).
In one case, a 58-year-old female with a documented allergy to topically applied cinnamic alcohol presented with eyelid dermatitis, which was found to be a manifestation of systemic contact dermatitis to cinnamon in the diet. Symptoms improved in two days and completely cleared five days after discontinuing the addition of cinnamon to food products (95599). In other case reports, two adults presented with allergic contact cheilitis following the ingestion of chai tea with cinnamon and yogurt with cinnamon. Cinnamon components were confirmed as the causative allergic agents with patch tests, and both cases of allergic contact cheilitis completely resolved upon cessation of the cinnamon-containing products (113516,113515).
Topically, allergic skin reactions and stomatitis from toothpaste flavored with cassia cinnamon have been reported (11915,11920). Intraoral allergic reactions with symptoms of tenderness and burning sensations of the oral mucosa have also been reported in patients using breath fresheners, toothpaste, mouthwash, candy, or chewing gum containing cinnamon, cinnamic aldehyde or cinnamic alcohol as flavoring agents. Glossodynia, or burning mouth syndrome, has also been reported in a 62-year-old female who ate apples dipped in cinnamon nightly (95598), and allergic contact dermatitis has been reported in a teenage female using a homemade cinnamon sugar face scrub (95596).
Endocrine ...In one clinical trial, a hypoglycemic seizure was reported in one patient taking cassia cinnamon 1 gram daily for 3 months. The event occurred one day after enrolling in the study (89648). It is unclear if cassia cinnamon caused this event.
Hepatic ...There is some concern about the safety of ingesting large amounts of cassia cinnamon for extended durations due to its coumarin content. Coumarin can cause hepatotoxicity in animal models (15299). In humans, very high doses of coumarin from 50-7000 mg/day can result in hepatotoxicity that resolves when coumarin is discontinued (15302). In clinical trials, taking cassia cinnamon 360 mg to 12 grams daily for 3 months did not significantly increase levels of aspartate transaminase (AST) or alanine transaminase (ALT) (21918,96280,108259). However, in one case report, acute hepatitis with elevated AST and ALT occurred in a 73-year-old female who started taking a cinnamon supplement (dose unknown) one week prior to admission. The cinnamon supplement was added on to high-dose rosuvastatin, which may have led to additive adverse hepatic effects. After discontinuing both products, liver function returned to normal, and the patient was able to restart rosuvastati without further complications (97249). In most cases, ingestion of cassia cinnamon won't provide a high enough amount of coumarin to cause significant toxicity; however, in especially sensitive people, such as those with liver disease or taking potentially hepatotoxic agents, prolonged ingestion of large amounts of cassia cinnamon might exacerbate the condition.
Immunologic ...An unspecified allergic reaction was reported in one patient taking cassia cinnamon 1 gram daily for 3 months (89648).
General
...Orally, Ceylon cinnamon is generally well tolerated, and adverse reactions are uncommon.
Most Common Adverse Effects:
Orally: Bloating, dyspepsia, nausea.
Topically: Allergic dermatitis, irritation of mucous membranes and skin.
Dermatologic
...Orally, a case of systemic contact dermatitis has been reported in a patient who consumed cinnamon (type not specified) after being previously sensitized to cinnamyl alcohol via cutaneous exposure (95599).
In a small study of oral Ceylon cinnamon, two patients reported itching (104520). In another small study, two patients reported rashes (108263).
Topically, cinnamon oil can cause skin irritation and allergic dermatitis, probably due to cinnamaldehyde which makes up 60% to 80% of cinnamon oil (2537,12635,92071,95596,95599). In one case report, a 16-year-old female experienced worsening dermatitis after using a homemade facial scrub containing cinnamon powder (type not specified). Symptoms improved after discontinuation of the scrub (95596). Several cases of intraoral allergic contact dermatitis have been reported in patients consuming cinnamon (type not specified) or using products containing constituents of cinnamon (95598).
Gastrointestinal ...Orally, gastrointestinal side effects such as heartburn, nausea, bloating, and dyspepsia have been reported (97250).
Hematologic ...Orally, a case of postoperative hemorrhage is reported in a 49-year-old patient after taking Ceylon cinnamon 1 tablespoon daily for 10 months. One day post-colectomy, the patient had an INR of 1.59 and intraabdominal bleeding that required exploratory laparotomies, blood transfusion, and fresh frozen plasma. Ultimately, the patient was discharged (112421).
Hepatic ...While there is concern about the coumarin content in cassia cinnamon increasing the risk for hepatic adverse effects and bleeding, the amount of coumarin in Ceylon cinnamon is negligible and unlikely to cause toxic effects (89652,92072,92073). In one case report, a 73-year-old female taking rosuvastatin for several months developed elevated liver function tests (LFTs), abdominal pain, nausea, and vomiting after taking cinnamon (unknown dose and type) for 7 days. The acute hepatitis and elevated LFTs resolved after stopping both cinnamon and rosuvastatin. The patient was later able to resume rosuvastatin without recurrence (97249).
General
...Orally, ginger is generally well tolerated.
However, higher doses of 5 grams per day increase the risk of side effects and reduce tolerability. Topically, ginger seems to be well tolerated.
Most Common Adverse Effects:
Orally: Abdominal discomfort, burping, diarrhea, heartburn, and a pepper-like irritant effect in the mouth and throat. However, some of these mild symptoms may be reduced by ingesting encapsulated ginger in place of powdered ginger.
Topically: Dermatitis in sensitive individuals.
Cardiovascular ...Orally, use of ginger resulted in mild arrhythmia in one patient in a clinical trial (16306).
Dermatologic
...Orally, ginger can cause hives (17933), as well as bruising and flushing (20316) or rash (20316).
Topically, ginger can cause dermatitis in sensitive individuals (12635,46902).
Gastrointestinal
...Orally, common side effects of ginger include nausea (17933,22602,89898,101761), belching (10380,103359), dry mouth (103359), dry retching (10380), vomiting (10380), burning sensation (10380), oral numbness (22602), abdominal discomfort (5343,89898,96253), heartburn (5343,7624,12472,16306,20316,51845,89894,89895,89898,89899)(101760,101761,101762,111543), diarrhea (5343,101760), constipation (89898,101760,101761), or a transient burning or "chilly hot" sensation of the tongue and throat (52076).
Orally, Number Ten, a specific product composed of rhubarb, ginger, astragalus, red sage, and turmeric, can increase the incidence of loose stools (20346).
Four cases of small bowel obstruction due to ginger bolus have been reported following the ingestion of raw ginger without sufficient mastication (chewing). In each case, the bolus was removed by enterotomy. Ginger is composed of cellulose and therefore is resistant to digestion. It can absorb water, which may cause it to swell and become lodged in narrow areas of the digestive tract (52115).
Genitourinary ...In one clinical trial, some patients reported increased menstrual bleeding while taking a specific ginger extract (Zintoma, Goldaru) 250 mg four times daily orally for 3 days (17931). An "intense" urge to urinate after 30 minutes was reported in two of eight patients given 0.5-1 gram of ginger (7624). However, this effect has not been corroborated elsewhere. Dysuria, flank pain, perineal pain, and urinary stream interruption have been reported in a 43-year-old male who drank ginger tea, containing 2-3 teaspoons of dry ginger, daily over 15 years. The adverse effects persisted for 4 years and were not associated with increases in urinary frequency or urgency. Upon discontinuing ginger, the patient's symptoms began to improve within one week and completely resolved after eight weeks, with no relapses six months later (107902).
Immunologic ...In one case report, a 59-year-old Japanese female with multiple allergic sensitivities developed pruritus and then anaphylactic shock after taking an oral ginger-containing herbal supplement for motion sickness (Keimei Gashinsan, Keimeido). The patient had used this supplement previously for over 20 years with no allergic reaction. The authors theorized the development of a cross-reactivity to ginger after the use of an oral supplement containing zedoary and turmeric, which are also in the Zingiberaceae family (102463).
Neurologic/CNS ...Orally, ginger may cause sedation, drowsiness, or dizziness (16306,17933,51845).
General
...Orally and intravaginally, kudzu seems to be well tolerated.
Serious Adverse Effects (Rare):
Orally: Elevated liver transaminases.
Cardiovascular ...Orally, side effects of kudzu reported in clinical trials have included palpitations and chest discomfort; however, these effects did not occur more frequently than with placebo (57924,57927).
Dermatologic ...Orally, a side effect of kudzu reported in one clinical trial has included urticaria; however, this effect did not occur more frequently than with placebo (57924). There is one case report of allergic reaction following use of a combination herbal product (Kakkonto) containing kudzu involving a maculopapular eruption starting on the thighs and spreading over the entire body (13111,57886).
Gastrointestinal ...Orally, some side effects of kudzu reported in clinical trials have included nausea, dyspepsia, and bloating; however, these effects did not occur more frequently than with placebo (57927,57942).
Genitourinary ...Intravaginally, irritation of the vulva has been reported with kudzu gel. These cases were generally mild and transient (110702).
Hematologic ...Intravenously, the kudzu derivative, puerarin, has caused intravascular hemolysis (13298,15025,57947).
Hepatic ...Orally, there are several cases reports of liver injury following use of kudzu involving elevated aspartate aminotransferase (AST) and alanine aminotransferase (ALT) levels (88777,92260).
Neurologic/CNS ...Orally, a side effect of kudzu reported in one clinical trial has included dizziness; however, this effect did not occur more frequently than with placebo (57924).
Other ...Orally, a side effect of kudzu reported in one clinical trial has included mastodynia; however, this effect did not occur more frequently than with placebo (57942).
General
...Orally, licorice is generally well tolerated when used in amounts commonly found in foods.
It seems to be well tolerated when licorice products that do not contain glycyrrhizin (deglycyrrhizinated licorice) are used orally and appropriately for medicinal purposes or when used topically, short-term.
Most Common Adverse Effects:
Orally: Headache, nausea, and vomiting.
Topically: Contact dermatitis.
Intravenously: Diarrhea, itching, nausea, and rash.
Serious Adverse Effects (Rare):
Orally: Case reports have raised concerns about acute renal failure, cardiac arrest, cardiac arrhythmias, hypertension, hypokalemia, muscle weakness, paralysis, pseudohyperaldosteronism, and seizure associated with long-term use or large amounts of licorice containing glycyrrhizin.
Cardiovascular
...Orally, excessive licorice ingestion can lead to pseudohyperaldosteronism, which can precipitate cardiovascular complications such as hypertension and hypertensive crisis, ventricular fibrillation or tachycardia, sinus pause, and cardiac arrest.
These effects are due to the licorice constituent glycyrrhizin and usually occur when 20-30 grams or more of licorice product is consumed daily for several weeks (781,15590,15592,15594,15596,15597,15599,15600,16835,97213) (104563,108574,108576,110305,112234). In one case report, an 89-year-old female taking an herbal medicine containing licorice experienced a fatal arrhythmia secondary to licorice-induced hypokalemia. The patient presented to the hospital with recurrent syncope, weakness, and fatigue for 5 days after taking an herbal medicine containing licorice for 2 months. Upon admission to the hospital, the patient developed seizures, QT prolongation, and ventricular arrhythmia requiring multiple defibrillations. Laboratory tests confirmed hypokalemia and pseudohyperaldosteronism (112234).
However, people with cardiovascular or kidney conditions may be more sensitive, so these adverse events may occur with doses as low as 5 grams of licorice product or glycyrrhizin 100 mg daily (15589,15593,15598,15600,59726). A case report in a 54-year-old male suggests that malnutrition might increase the risk of severe adverse effects with excessive licorice consumption. This patient presented to the emergency room with cardiac arrest and ventricular fibrillation after excessive daily consumption of licorice for about 3 weeks. This caused pseudohyperaldosteronism and then hypokalemia, leading to cardiovascular manifestations. In spite of resuscitative treatment, the patient progressed to kidney failure, refused dialysis, and died shortly thereafter (103791).
Dermatologic
...There have been reports of contact allergy, resulting in an itchy reddish eruption, occurring in patients that applied cosmetic products containing oil-soluble licorice extracts (59912).
There have also been at least 3 cases of allergic contact dermatitis reported with the topical application of glycyrrhizin-containing products to damaged skin. In one case report, a 31-year-old female with acne presented with a 2-year history of pruritic erythematous-scaly plaques located predominantly on the face and neck after the use of a cosmetic product containing licorice root extract 1%. The patient had a positive skin patch test to licorice root extract, leading the clinicians to hypothesize that the use of benzoyl peroxide, a strong irritant, might have sensitized the patient to licorice (108578). Burning sensation, itching, redness, and scaling were reported rarely in patients applying a combination of licorice, calendula, and snail secretion filtrate to the face. The specific role of licorice is unclear (110322).
In rare cases, the glycyrrhizin constituent of licorice has caused rash and itching when administered intravenously (59712).
Endocrine
...Orally, excessive licorice ingestion can cause a syndrome of apparent mineralocorticoid excess, or pseudohyperaldosteronism, with sodium and water retention, increased urinary potassium loss, hypokalemia, and metabolic alkalosis due to its glycyrrhizin content (781,10619,15591,15592,15593,15594,15595,15596,15597,15598)(15600,16057,16835,25659,25660,25673,25719,26439,59818,59822)(59832,59864,91722,104563,108568,108574,110305,112234).
These metabolic abnormalities can lead to hypertension, edema, EKG changes, fatigue, syncope, arrhythmias, cardiac arrest, headache, lethargy, muscle weakness, dropped head syndrome (DHS), rhabdomyolysis, myoglobinuria, paralysis, encephalopathy, respiratory impairment, hyperparathyroidism, and acute kidney failure (10393,10619,15589,15590,15593,15594,15596,15597,15599)(15600,16057,16835,25660,25673,25719,26439,31562,59709,59716)(59720,59740,59787,59820,59826,59882,59889,59900,91722,97214,100522) (104563,108576,108577). These effects are most likely to occur when 20-30 grams of licorice products containing glycyrrhizin 400 mg or more is consumed daily for several weeks (781,15590,15592,15594,15596,15597,15599,15600,16835,108574). However, some people may be more sensitive, especially those with hypertension, diabetes, heart problems, or kidney problems (15589,15593,15598,15600,59726,108576,108577) and even low or moderate consumption of licorice may cause hypertensive crisis or hypertension in normotensive individuals (1372,97213). The use of certain medications with licorice may also increase the risk of these adverse effects (108568,108577). One case report determined that the use of large doses of licorice in an elderly female stabilized on fludrocortisone precipitated hypokalemia and hypertension, requiring inpatient treatment (108568). Another case report describes severe hypokalemia necessitating intensive care treatment due to co-ingestion of an oral glycyrrhizin-specific product and hydrochlorothiazide for 1 month (108577). Glycyrrhetinic acid has a long half-life, a large volume of distribution, and extensive enterohepatic recirculation. Therefore, it may take 1-2 weeks before hypokalemia resolves (781,15595,15596,15597,15600). Normalization of the renin-aldosterone axis and blood pressure can take up to several months (781,15595,108568). Treatment typically includes the discontinuation of licorice, oral and intravenous potassium supplementation, and short-term use of aldosterone antagonists, such as spironolactone (108574,108577).
Chewing tobacco flavored with licorice has also been associated with toxicity. Chewing licorice-flavored tobacco, drinking licorice tea, or ingesting large amounts of black licorice flavored jelly beans or lozenges has been associated with hypertension and suppressed renin and aldosterone levels (12671,12837,97214,97215,97217,108574). One case report suggests that taking a combination product containing about 100 mg of licorice and other ingredients (Jintan, Morishita Jintan Co.) for many decades may be associated with hypoaldosteronism, even up to 5 months after discontinuation of the product (100522). In another case report, licorice ingestion led to hyperprolactinemia in a female (59901). Licorice-associated hypercalcemia has also been noted in a case report (59766).
Gastrointestinal ...Nausea and vomiting have been reported rarely following oral use of deglycyrrhizinated licorice (25694,59871). Intravenously, the glycyrrhizin constituent of licorice has rarely caused gastric discomfort, diarrhea, or nausea (59712,59915).
Immunologic ...There have been reports of contact allergy, resulting in an itchy reddish eruption, occurring in patients that applied cosmetic products containing oil-soluble licorice extracts (59912). There have also been at least 3 cases of allergic contact dermatitis reported with the topical application of glycyrrhizin-containing products to damaged skin. In one case report, a 31-year-old female with acne presented with a 2-year history of pruritic erythematous-scaly plaques located predominantly on the face and neck after the use of a cosmetic product containing licorice root extract 1%. The patient had a positive skin patch test to licorice root extract, leading the clinicians to hypothesize that the use of benzoyl peroxide, a strong irritant, might have sensitized the patient to licorice (108578).
Musculoskeletal ...In a case report, excessive glycyrrhizin-containing licorice consumption led to water retention and was thought to trigger neuropathy and carpal tunnel syndrome (59791).
Neurologic/CNS ...Orally, licorice containing larger amounts of glycyrrhizin may cause headaches. A healthy woman taking glycyrrhizin 380 mg daily for 2 weeks experienced a headache (59892). Intravenously, the glycyrrhizin constituent of licorice has rarely caused headaches or fatigue (59721). In a case report, licorice candy ingestion was associated with posterior reversible encephalopathy syndrome accompanied by a tonic-clonic seizure (97218).
Ocular/Otic ...Orally, consuming glycyrrhizin-containing licorice 114-909 grams has been associated with transient visual loss (59714).
Pulmonary/Respiratory ...Orally, large amounts of licorice might lead to pulmonary edema. In one case report, a 64-year old male consumed 1020 grams of black licorice (Hershey Twizzlers) containing glycyrrhizin 3.6 grams over 3 days, which resulted in pulmonary edema secondary to pseudohyperaldosteronism (31561). Intravenously, the glycyrrhizin constituent of licorice has caused cold or flu-like symptoms, although these events are not common (59712,59721).
General ...Orally, lobelia can cause nausea, vomiting, diarrhea, coughing, dizziness, tremors, and throat irritation. These adverse effects have been reported with doses as low as 50 mg (4,16414). Lobelia leaf can cause toxicity when taken in doses of 600 mg or higher. Symptoms of lobelia toxicity include sweating, tachycardia, convulsions, hypothermia, hypotension, coma, and death (4,11).
Cardiovascular ...Orally, high doses of lobelia leaf can cause toxicity. Symptoms of lobelia toxicity include tachycardia, hypotension, and death (4,11).
Gastrointestinal ...Orally, lobelia can cause nausea, vomiting, diarrhea, and throat irritation (4,16414).
Neurologic/CNS ...Orally, lobelia can cause dizziness and tremors. High doses of lobelia leaf can cause toxicity. Symptoms of lobelia toxicity include convulsions, coma, and death (4,11).
Pulmonary/Respiratory ...Orally, lobelia can cause coughing and throat irritation (4,16414).
Other ...Orally, high doses of lobelia leaf can cause toxicity resulting in death. Toxicity has been reported to occur at doses as low as 600 mg, with doses of 4000 mg or more considered to be fatal (4,11).
General
...Orally, magnolia seems to be well tolerated.
Most Common Adverse Effects:
Topically: Contact dermatitis.
Dermatologic ...Topically, magnolia bark has been associated with reports of allergic contact dermatitis (92463,92468,95030,110709). In several cases, the use of anti-aging facial creams containing magnolia bark extract was associated with allergic contact dermatitis of the face (92463,92468,95030). In one case, the use of a vaginal gel containing magnolia bark extract was associated with allergic contact dermatitis of the vulva (110709). Symptoms typically resolve with the use of topical corticosteroids and discontinuation of magnolia bark extract (95030,110709). Patch testing suggests that the magnolia bark extract constituents magnolol and honokiol are responsible for this adverse effect (110709).
Endocrine ...In a clinical trial of an oral combination product containing extracts of magnolia and phellodendron, one patient reported thyroid dysfunction (14349). However, it's not known if this side effect is related to magnolia or some other factor.
Gastrointestinal ...In a clinical trial of an oral combination product containing extracts of magnolia and phellodendron, one patient reported heartburn (14349). However, it's not known if this side effect is related to magnolia or some other factor.
Neurologic/CNS ...In a clinical trial of an oral combination product containing extracts of magnolia and phellodendron, one patient reported shaking hands and perilabial numbness. Another patient reported fatigue and headache (14349). However, it's not known if these side effects are related to magnolia or some other factor.
Psychiatric ...In a clinical trial of an oral combination product containing extracts of magnolia and phellodendron, one patient reported sexual dysfunction (14349). However, it's not known if this side effect is related to magnolia or some other factor.
General
...Orally, peony seems to be well tolerated when used alone and as part of Chinese herbal formulas.
Most Common Adverse Effects:
Orally: Abdominal distension, anorexia, diarrhea, gastrointestinal discomfort, nausea.
Topically: Dermatitis.
Dermatologic ...Topically, peony has been reported to cause contact dermatitis (13555).
Endocrine ...Orally, a specific traditional Chinese medicine preparation called DDT has been reported to lower follicle-stimulating hormone (FSH) levels and increase estradiol levels. It is not known if this effect is due to peony or the other ingredients (48404). Another specific traditional Chinese medicine preparation, Toki-shakuyaku-san, has been reported to increase plasma progesterone levels in some patients. It is not known if this effect is due to peony or the other ingredients (15294).
Gastrointestinal ...Orally, peony and total glucosides of peony (TGP) have been reported to cause gastrointestinal discomfort, including abdominal distension, anorexia, diarrhea, and nausea, in some patients (13538,92785,97949,98466,100992). In one clinical study, diarrhea was reported in 5% of patients taking TGP 600 mg three times daily for 24 weeks versus 1% of patients taking placebo (100992).
Hematologic ...Orally, there is one case report of easy gum bleeding, epistaxis, and skin bruising with an international normalized ratio (INR) above 6 in a 61-year-old male who was previously stable on warfarin therapy. This patient had switched from one brand of quilinggao, a popular Chinese herbal product, to another brand 5 days prior. This product contained Fritillaria spp. (beimu), Paeonia rubra, Chinese peony (chishao), Lonicera japonica (jinyinhua), and Poncirus trifoliata (jishi). The patient's INR decreased to 1.9 after temporary withdrawal of warfarin therapy. Upon re-initiation of quilinggao, his INR increased to 5.2. It is not known if the increased INR is due to peony or the other ingredients (68343).
General
...Orally, schisandra seems to be generally well tolerated.
Most Common Adverse Effects:
Orally: Decreased appetite, heartburn, stomach upset, and urticaria.
Dermatologic ...Orally, schisandra can cause urticaria in some patients (11).
Gastrointestinal ...Orally, schisandra can cause heartburn, decreased appetite, and stomach upset (11).
